A phase I study to assess the mass balance, excretion, and pharmacokinetics of [14C]-ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors
- PMID: 28932928
- PMCID: PMC5948259
- DOI: 10.1007/s10637-017-0509-1
A phase I study to assess the mass balance, excretion, and pharmacokinetics of [14C]-ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors
Abstract
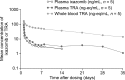
This two-part, phase I study evaluated the mass balance, excretion, pharmacokinetics (PK), and safety of ixazomib in patients with advanced solid tumors. In Part A of the study, patients received a single 4.1 mg oral solution dose of [14C]-ixazomib containing ~500 nCi total radioactivity (TRA), followed by non-radiolabeled ixazomib (4 mg capsule) on days 14 and 21 of the 35-day PK cycle. Patients were confined to the clinic for the first 168 h post dose and returned for 24 h overnight clinic visits on days 14, 21, 28, and 35. Blood, urine, and fecal samples were collected during Part A to assess the mass balance (by accelerator mass spectrometry), excretion, and PK of ixazomib. During Part B of the study, patients received non-radiolabeled ixazomib (4 mg capsules) on days 1, 8, and 15 of 28-day cycles. After oral administration, ixazomib was rapidly absorbed with a median plasma Tmax of 0.5 h and represented 70% of total drug-related material in plasma. The mean total recovery of administered TRA was 83.9%; 62.1% in urine and 21.8% in feces. Only 3.23% of the administered dose was recovered in urine as unchanged drug up to 168 h post dose, suggesting that most of the TRA in urine was attributable to metabolites. All patients experienced a treatment-emergent adverse event, which most commonly involved the gastrointestinal system. These findings suggest that ixazomib is extensively metabolized, with urine representing the predominant route of excretion of drug-related material.Trial ID: ClinicalTrials.gov # NCT01953783.
Keywords: ADME; AMS; Ixazomib; Mass balance; Pharmacokinetics; Total radioactivity.
Conflict of interest statement
Conflicts of interest
NG, SZ, SP, MP, SC, MJH, BW, CX, XZ, and KV are employees of Millennium Pharmaceuticals, Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. DRS has no conflicts of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


References
-
- Kupperman E, Lee EC, Cao Y, Bannerman B, Fitzgerald M, Berger A, Yu J, Yang Y, Hales P, Bruzzese F, Liu J, Blank J, Garcia K, Tsu C, Dick L, Fleming P, Yu L, Manfredi M, Rolfe M, Bolen J. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70(5):1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. - DOI - PubMed
-
- Assouline SE, Chang J, Cheson BD, Rifkin R, Hamburg S, Reyes R, Hui AM, Yu J, Gupta N, Di BA, Shou Y, Martin P. Phase 1 dose-escalation study of IV ixazomib, an investigational proteasome inhibitor, in patients with relapsed/refractory lymphoma. Blood Cancer J. 2014;4:e251. doi: 10.1038/bcj.2014.71. - DOI - PMC - PubMed
-
- Gupta N, Goh YT, Min CK, Lee JH, Kim K, Wong RS, Chim CS, Hanley MJ, Yang H, Venkatakrishnan K, Hui AM, Esseltine DL, Chng WJ. Pharmacokinetics and safety of ixazomib plus lenalidomide-dexamethasone in Asian patients with relapsed/refractory myeloma: a phase 1 study. J Hematol Oncol. 2015;8:103. doi: 10.1186/s13045-015-0198-1. - DOI - PMC - PubMed
-
- Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, Stewart AK, Hari P, Roy V, Vescio R, Kaufman JL, Berg D, Liao E, Di BA, Estevam J, Gupta N, Hui AM, Rajkumar V, Richardson PG. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503–1512. doi: 10.1016/S1470-2045(14)71125-8. - DOI - PubMed
-
- Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, Hui AM, Gupta N, Di BA, Yu J, Shou Y, Niesvizky R. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124(7):1047–1055. doi: 10.1182/blood-2014-01-548941. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

