Methodological Considerations for Comparison of Brand Versus Generic Versus Authorized Generic Adverse Event Reports in the US Food and Drug Administration Adverse Event Reporting System (FAERS)
- PMID: 28933038
- PMCID: PMC5842081
- DOI: 10.1007/s40261-017-0574-4
Methodological Considerations for Comparison of Brand Versus Generic Versus Authorized Generic Adverse Event Reports in the US Food and Drug Administration Adverse Event Reporting System (FAERS)
Abstract
Background: The US Food and Drug Administration Adverse Event Reporting System (FAERS), a post-marketing safety database, can be used to differentiate brand versus generic safety signals.
Objective: To explore the methods for identifying and analyzing brand versus generic adverse event (AE) reports.
Methods: Public release FAERS data from January 2004 to March 2015 were analyzed using alendronate and carbamazepine as examples. Reports were classified as brand, generic, and authorized generic (AG). Disproportionality analyses compared reporting odds ratios (RORs) of selected known labeled serious adverse events stratifying by brand, generic, and AG. The homogeneity of these RORs was compared using the Breslow-Day test. The AG versus generic was the primary focus since the AG is identical to brand but marketed as a generic, therefore minimizing generic perception bias. Sensitivity analyses explored how methodological approach influenced results.
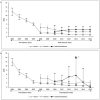
Results: Based on 17,521 US event reports involving alendronate and 3733 US event reports involving carbamazepine (immediate and extended release), no consistently significant differences were observed across RORs for the AGs versus generics. Similar results were obtained when comparing reporting patterns over all time and just after generic entry. The most restrictive approach for classifying AE reports yielded smaller report counts but similar results.
Conclusion: Differentiation of FAERS reports as brand versus generic requires careful attention to risk of product misclassification, but the relative stability of findings across varying assumptions supports the utility of these approaches for potential signal detection.
Conflict of interest statement
Figures





References
-
- [Accessed November 28, 2016];Generic Pharmaceutical Association (GPhA) 2016 Generic Drug Savings & Access in the United States Report. http://www.gphaonline.org/media/generic-drug-savings-2016/index.html.
-
- Crawford P, et al. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure. 2006;15(3):165–76. - PubMed
-
- Erickson SC, et al. Clinical and pharmacy utilization outcomes with brand to generic antiepileptic switches in patients with epilepsy. Epilepsia. 2011;52(7):1365–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

