Milk's Role as an Epigenetic Regulator in Health and Disease
- PMID: 28933365
- PMCID: PMC5456335
- DOI: 10.3390/diseases5010012
Milk's Role as an Epigenetic Regulator in Health and Disease
Abstract
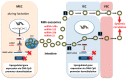
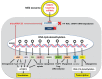
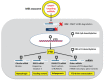
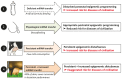
It is the intention of this review to characterize milk's role as an epigenetic regulator in health and disease. Based on translational research, we identify milk as a major epigenetic modulator of gene expression of the milk recipient. Milk is presented as an epigenetic "doping system" of mammalian development. Milk exosome-derived micro-ribonucleic acids (miRNAs) that target DNA methyltransferases are implicated to play the key role in the upregulation of developmental genes such as FTO, INS, and IGF1. In contrast to miRNA-deficient infant formula, breastfeeding via physiological miRNA transfer provides the appropriate signals for adequate epigenetic programming of the newborn infant. Whereas breastfeeding is restricted to the lactation period, continued consumption of cow's milk results in persistent epigenetic upregulation of genes critically involved in the development of diseases of civilization such as diabesity, neurodegeneration, and cancer. We hypothesize that the same miRNAs that epigenetically increase lactation, upregulate gene expression of the milk recipient via milk-derived miRNAs. It is of critical concern that persistent consumption of pasteurized cow's milk contaminates the human food chain with bovine miRNAs, that are identical to their human analogs. Commercial interest to enhance dairy lactation performance may further increase the epigenetic miRNA burden for the milk consumer.
Keywords: DNA methyltransferase; FTO; breastfeeding; epigenetic regulation; exosome; infant formula; lactation; miRNA-148a; milk; non-communicalbe diseases of civilization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

