Carnosine suppresses oxygen-glucose deprivation/recovery-induced proliferation and migration of reactive astrocytes of rats in vitro
- PMID: 28933425
- PMCID: PMC5758661
- DOI: 10.1038/aps.2017.126
Carnosine suppresses oxygen-glucose deprivation/recovery-induced proliferation and migration of reactive astrocytes of rats in vitro
Abstract
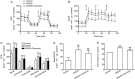
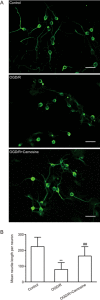
Glial scar formation resulted from excessive astrogliosis limits axonal regeneration and impairs recovery of function, thus an intervention to ameliorate excessive astrogliosis is crucial for the recovery of neurological function after cerebral ischemia. In this study we investigated the effects of carnosine, an endogenous water-soluble dipeptide (β-alanyl-L-histidine), on astrogliosis of cells exposed to oxygen-glucose deprivation/recovery (OGD/R) in vitro. Primary cultured rat astrocytes exhibited a significant increase in proliferation at 24 h recovery after OGD for 2 h. Pretreatment with carnosine (5 mmol/L) caused G1 arrest of reactive astrocytes, significantly attenuated OGD/R-induced increase in cyclin D1 protein expression and suppressed OGD/R-induced proliferation of reactive astrocytes. Carnosine treatment also reversed glycolysis and ATP production, which was elevated at 24 h recovery after OGD. A marked increase in migration of reactive astrocytes was observed at 24 h after OGD, whereas carnosine treatment reversed the expression levels of MMP-9 and suppressed the migration of astrocytes. Furthermore, carnosine also improved neurite growth of cortical neurons co-cultured with astrocytes under ischemic conditions. These results demonstrate that carnosine may be a promising candidate for inhibiting astrogliosis and promoting neurological function recovery after ischemic stroke.
Figures




References
-
- Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia 2014; 62: 1895–904. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

