Anti-IL5 therapies for asthma
- PMID: 28933516
- PMCID: PMC6483800
- DOI: 10.1002/14651858.CD010834.pub3
Anti-IL5 therapies for asthma
Update in
-
Anti-IL-5 therapies for asthma.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD010834. doi: 10.1002/14651858.CD010834.pub4. Cochrane Database Syst Rev. 2022. PMID: 35838542 Free PMC article.
Abstract
Background: This review is the first update of a previously published review in The Cochrane Library (Issue 7, 2015). Interleukin-5 (IL-5) is the main cytokine involved in the activation of eosinophils, which cause airway inflammation and are a classic feature of asthma. Monoclonal antibodies targeting IL-5 or its receptor (IL-5R) have been developed, with recent studies suggesting that they reduce asthma exacerbations, improve health-related quality of life (HRQoL) and lung function. These are being incorporated into asthma guidelines.
Objectives: To compare the effects of therapies targeting IL-5 signalling (anti-IL-5 or anti-IL-5Rα) with placebo on exacerbations, health-related qualify of life (HRQoL) measures, and lung function in adults and children with chronic asthma, and specifically in those with eosinophilic asthma refractory to existing treatments.
Search methods: We searched the Cochrane Airways Trials Register, clinical trials registries, manufacturers' websites, and reference lists of included studies. The most recent search was March 2017.
Selection criteria: We included randomised controlled trials comparing mepolizumab, reslizumab and benralizumab versus placebo in adults and children with asthma.
Data collection and analysis: Two authors independently extracted data and analysed outcomes using a random-effects model. We used standard methods expected by Cochrane.
Main results: Thirteen studies on 6000 participants met the inclusion criteria. Four used mepolizumab, four used reslizumab, and five used benralizumab. One study in benralizumab was terminated early due to sponsor decision and contributed no data. The studies were predominantly on people with severe eosinophilic asthma, which was similarly but variably defined. Eight included children over 12 years but these results were not reported separately. We deemed the risk of bias to be low, with all studies contributing data being of robust methodology. We considered the quality of the evidence for all comparisons to be high overall using the GRADE scheme, with the exception of intravenous mepolizumab because this is not currently a licensed delivery route.All of the anti-IL-5 treatments assessed reduced rates of 'clinically significant' asthma exacerbation (defined by treatment with systemic corticosteroids for three days or more) by approximately half in participants with severe eosinophilic asthma on standard of care (at least medium-dose inhaled corticosteroids (ICS)) with poorly controlled disease (either two or more exacerbations in the preceding year or Asthma Control Questionnaire (ACQ) 1.5 or more). Non-eosinophilic participants treated with benralizumab also showed a significant reduction in exacerbation rates, but no data were available for non-eosinophilic participants, and mepolizumab or reslizumab.We saw modest improvements in validated HRQoL scores with all anti-IL-5 agents in severe eosinophilic asthma. However these did not exceed the minimum clinically important difference for ACQ and Asthma Quality of Life Questionnaire (AQLQ), with St. George's Respiratory Questionnaire (SGRQ) only assessed in two studies. The improvement in HRQoL scores in non-eosinophilic participants treated with benralizumab, the only intervention for which data were available in this subset, was not statistically significant, but the test for subgroup difference was negative.All anti-IL-5 treatments produced a small but statistically significant improvement in mean pre-bronchodilator forced expiratory flow in one second (FEV1) of between 0.08 L and 0.11 L.There were no excess serious adverse events with any anti-IL-5 treatment, and indeed a reduction in favour of mepolizumab that could be due to a beneficial effect on asthma-related serious adverse events. There was no difference compared to placebo in adverse events leading to discontinuation with mepolizumab or reslizumab, but significantly more discontinued benralizumab than placebo, although the absolute numbers were small (36/1599 benralizumab versus 9/998 placebo).Mepolizumab, reslizumab and benralizumab all markedly reduced blood eosinophils, but benralizumab resulted in almost complete depletion, whereas a small number remained with mepolizumab and reslizumab. The implications for efficacy and/or adverse events are unclear.
Authors' conclusions: Overall our study supports the use of anti-IL-5 treatments as an adjunct to standard of care in people with severe eosinophilic asthma and poor control. These treatments roughly halve the rate of asthma exacerbations in this population. There is limited evidence for improved HRQoL scores and lung function, which may not meet clinically detectable levels. There were no safety concerns regarding mepolizumab or reslizumab, and no excess serious adverse events with benralizumab, although there remains a question over adverse events significant enough to prompt discontinuation.Further research is needed on biomarkers for assessing treatment response, optimal duration and long-term effects of treatment, risk of relapse on withdrawal, non-eosinophilic patients, children (particularly under 12 years), and comparing anti-IL-5 treatments to each other and, in people eligible for both, to anti-immunoglobulin E. For benralizumab, future studies should closely monitor rates of adverse events prompting discontinuation.
Conflict of interest statement
HF: none known.
AW: none known.
CP: none known.
LB: none known.
SM: none known.
Figures

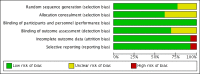











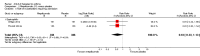


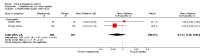















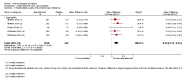





Update of
-
Mepolizumab versus placebo for asthma.Cochrane Database Syst Rev. 2015 Jul 27;(7):CD010834. doi: 10.1002/14651858.CD010834.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Sep 21;9:CD010834. doi: 10.1002/14651858.CD010834.pub3. PMID: 26214266 Updated.
References
References to studies included in this review
-
- Bjermer L, Lemiere C, Maspero J, Ciesielska M, O'Brien C, Zangrilli J. A randomized phase 3 study of the efficacy and safety of reslizumab in subjects with asthma with elevated eosinophils. European Respiratory Journal 2014;44(Suppl 58):P299. [CENTRAL: 1053372; CRS: 4900126000028560; EMBASE: 71849984]
- Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016;150(4):789‐98. [CENTRAL: 1139859 ; CRS: 4900132000017682; PUBMED: 27056586] - PubMed
- Maspero J, Bjermer L, Lemiere C, Ciesielska M, O'Brien C, Zangrilli J. A randomized phase 3 study assessing patient reported outcomes and safety of reslizumab in patients with asthma with elevated eosinophils. Annals of Allergy, Asthma and Immunology 2014;113(5 SUPPL. 1):A21. [CENTRAL: 1020022; CRS: 4900126000021720; EMBASE: 71679175]
- NCT01270464. A study to evaluate the efficacy and safety of reslizumab (0.3 or 3.0 mg/kg) as treatment for patients (12‐75 years of age) with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01270464 (first received 29 December 2010).
-
- Bleecker E, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Benralizumab provides significant improvements for patients with severe, uncontrolled asthma: SIROCCO Phase III results. European Respiratory Journal 2016;48:OA4832.
- Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting beta2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet 2016;388(10056):2115–27. - PubMed
- NCT01928771. Efficacy and safety study of benralizumab added to high‐dose inhaled corticosteroid plus LABA in patients with uncontrolled asthma. clinicaltrials.gov/show/NCT01928771 (first received 16 August 2013). [CRS: 4900132000027654]
-
- Castro M, Gossage DL, Ward CK, Wu Y, Khatri DB, Molfino NA, et al. Benralizumab reduces exacerbations and improves lung function in adults with uncontrolled eosinophilic asthma. American Journal of Respiratory and Critical Care Medicine 2014;189:B101. [CENTRAL: 1131488; CRS: 4900132000009717; EMBASE: 72043211]
- Castro M, Wenzel S, Kolbeck R, Khatry D, Christine W, Wu Y, et al. A phase 2 study of benralizumab on exacerbations, lung function, and asthma control in adults with uncontrolled eosinophilic asthma. European Respiratory Journal 2014;44(Suppl 58):2909. [CENTRAL: 1053384; CRS: 4900126000028573]
- Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti‐interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose‐ranging study. Lancet Respiratory Medicine 2014;2(11):879‐9. [PUBMED: 25306557] - PubMed
- Eck S, Castro M, Sinibaldi D, White W, Folliot K, Gossage D, et al. Benralizumab effect on blood basophil counts in adults with uncontrolled asthma. European Respiratory Journal 2014;44(Suppl 58):297. [CENTRAL: 1053403; CRS: 4900126000028594; EMBASE: 71849982]
- NCT01238861. Study to evaluate the efficacy and safety of MEDI‐563 in adults with uncontrolled asthma. clinicaltrials.gov/ct2/show/NCT01238861 (first received 9 November 2010).
- Wang B, Yan L, Hutmacher M, White WI, Ward CK, Nielsen J, et al. Exposure‐response analysis for determination of benralizumab optimal dosing regimen in adults with asthma. American Journal of Respiratory and Critical Care Medicine 2014;189:A1324. [CENTRAL: 1035648; CRS: 4900126000023160; EMBASE: 72043774]
-
- Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late‐onset asthma and elevated blood eosinophils. Pulmonary Pharmacology and Therapeutics 2017;43:39‐45. - PubMed
- Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(4):e15. [CRS: 4900126000028793; DOI: 10.1016/S2213-2600(15)00042-9; EMBASE: 2015833476] - DOI - PubMed
- Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(5):355‐66. - PubMed
- NCT01287039. A study to evaluate the efficacy and safety of reslizumab (3.0 mg/kg) in the reduction of clinical asthma exacerbations in patients (12‐75 years of age) with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01287039 (first received 28 January 2011).
-
- Castro M, Zangrilli J, Wechsler ME. Corrections to Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(4):e15. [CRS: 4900126000028793; EMBASE: 2015833476] - PubMed
- Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respiratory Medicine 2015;3(5):355‐66. - PubMed
- NCT01285323. A study to evaluate the efficacy and safety of reslizumab in patients with eosinophilic asthma. clinicaltrials.gov/ct2/show/NCT01285323 (first received 25 January 2011).
References to studies excluded from this review
-
- Albers F, Cockle S, Gunsoy N, Shin JY, Nelsen L, Muellerova H. Eligibility for mepolizumab, omalizumab and reslizumab in the EU population: The IDEAL study [PA4216]. European Respiratory Society 26thAnnual Congress; 2016 Sep 3‐7; London. 2016.
-
- Alvarez‐Cuesta E, Cuesta‐Herranz J, Puyana‐Ruiz J, Cuesta‐Herranz C, Blanco‐Quiros A. Monoclonal antibody‐standardized cat extract immunotherapy: risk‐benefit effects from a double‐blind placebo study. Journal of Allergy and Clinical Immunology 1994;93(3):556‐66. [] - PubMed
-
- Armentia A, Arranz M, Martin JM, Fuente R, Sanchez P, Barber D, et al. Evaluation of immune complexes after immunotherapy with wheat flour in bakers' asthma. Annals of Allergy 1992;69(5):441‐4. [] - PubMed
-
- Austin D, Pouliquen I, Keene O, Yancey S. Blood eosinophil dose response to oral corticosteroids in a population of patients with severe asthma [PA1110]. European Respiratory Society 26th Annual Congress; 2016 Sep 3‐7; London. 2016.
-
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti‐immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate‐to‐severe) allergic asthma. Allergy 2004;59(7):701‐8. [] - PubMed
References to ongoing studies
-
- EUCTR2005‐001932‐61‐GB. Mepolizumab and exacerbation frequency in refractory eosinophilic asthma. A randomised, double blind, placebo controlled, parallel group trial. clinicaltrialsregister.eu/ctr‐search/search?query=EUCTR2005‐001932‐61‐GB (first received 16 November 2005).
-
- NCT01520051. Mepolizumab treatment for rhinovirus‐induced asthma exacerbations (MATERIAL) [The efficacy of mepolizumab treatment on rhinovirus induced asthma exacerbations]. clinicaltrials.gov/show/NCT01520051 (first received 25 January 2012). []
-
- NCT02452190. Study of reslizumab in patients with uncontrolled asthma and elevated blood eosinophils. clinicaltrials.gov/show/NCT02452190 (first received 13 May 2015). [CRS: 4900132000027647]
-
- NCT02555371. Cessation versus continuation of long‐term mepolizumab in severe eosinophilic asthma patients. clinicaltrials.gov/show/NCT02555371 (first received 17 September 2015). [CRS: 4900132000027646]
-
- NCT02594332. Effects of mepolizumab compared to placebo on airway physiology in patients with eosinophilic asthma: MEMORY study (MEMORY). clinicaltrials.gov/show/NCT02594332 (first received 31 August 2015). [CRS: 4900132000027645]
Additional references
-
- Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nature Medicine 2013;19(8):977‐9. [PUBMED: 23921745] - PubMed
-
- Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI‐563, an anti‐IL‐5 receptor alpha antibody, in a phase I study of subjects with mild asthma. Journal of Allergy and Clinical Immunology 2010;125(6):1237‐1244.e2. [PUBMED: 20513521] - PubMed
-
- Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti‐interleukin‐5 therapies in patients with severe asthma: global and indirect meta‐analyses of randomized placebo‐controlled trials. Clinical and Experimental Allergy 2017;47(1):129‐38. [PUBMED: 27859832] - PubMed
-
- Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet 2015;386(9998):1086‐96. [PUBMED: 26383000] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

