Immune responses after live attenuated influenza vaccination
- PMID: 28933664
- PMCID: PMC5861782
- DOI: 10.1080/21645515.2017.1377376
Immune responses after live attenuated influenza vaccination
Abstract
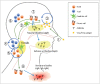
Since 2003 (US) and 2012 (Europe) the live attenuated influenza vaccine (LAIV) has been used as an alternative to the traditional inactivated influenza vaccines (IIV). The immune responses elicted by LAIV mimic natural infection and have been found to provide broader clinical protection in children compared to the IIVs. However, our knowledge of the detailed immunological mechanisims induced by LAIV remain to be fully elucidated, and despite 14 years on the global market, there exists no correlate of protection. Recently, matters are further complicated by differing efficacy data from the US and Europe which are not understood. Better understanding of the immune responses after LAIV may aid in achieving the ultimate goal of a future "universal influenza vaccine". In this review we aim to cover the current understanding of the immune responses induced after LAIV.
Keywords: Antibody; T-cell; immune response; live attenuated influenza vaccine (LAIV).
Figures
References
-
- WHO Fact sheet about seasonal influenza. 2017. http://www.who.int/mediacentre/factsheets/fs211/en/
-
- WHO Vaccines against influenza WHo position paper – November 2012. Weekly Epidemiological Record. 2012;87(47):461-476. PMID:23210147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
