Nasal aluminum (oxy)hydroxide enables adsorbed antigens to induce specific systemic and mucosal immune responses
- PMID: 28933668
- PMCID: PMC5703361
- DOI: 10.1080/21645515.2017.1365995
Nasal aluminum (oxy)hydroxide enables adsorbed antigens to induce specific systemic and mucosal immune responses
Abstract
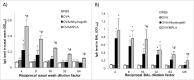
Some insoluble aluminum salts are commonly used in injectable vaccines as adjuvants to accelerate, prolong, or enhance the antigen-specific immune responses. Data from previous studies testing the nasal mucosal vaccine adjuvant activity of aluminum salts are conflicting. The present study is designed to further assess the feasibility of using aluminum salts in injectable vaccines as nasal mucosal vaccine adjuvants. Using Alhydrogel®, the international scientific standard of aluminum (oxy)hydroxide gels, and ovalbumin or 3 × M2e-HA2, a synthetic influenza virus fusion protein, as antigens, we showed in a mouse model that when dosed intranasally Alhydrogel® enables antigens adsorbed on it to induce stronger antigen-specific immune responses in both serum samples (e.g., specific IgG) and nasal and lung mucosal secretions (i.e., specific IgA) in all immunized mice, as compared with nasal immunization with the antigens alone. Rerouting insoluble aluminum salts in injectable vaccines may represent a viable approach for (nasal) mucosal vaccine adjuvant discovery.
Keywords: MPLA; Mucosal vaccine adjuvant; aluminum salts; antibody responses; cytokine release.
Figures


Similar articles
-
Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine.J Control Release. 2018 Dec 28;292:111-118. doi: 10.1016/j.jconrel.2018.10.020. Epub 2018 Oct 16. J Control Release. 2018. PMID: 30339906 Free PMC article.
-
Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration.Mol Pharm. 2020 Sep 8;17(9):3259-3269. doi: 10.1021/acs.molpharmaceut.0c00372. Epub 2020 Aug 27. Mol Pharm. 2020. PMID: 32787271
-
The ovine nasal mucosa: an alternative tissue site for mucosal immunization.Methods. 2006 Feb;38(2):112-6. doi: 10.1016/j.ymeth.2005.09.010. Methods. 2006. PMID: 16427306
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants.Vaccine. 2002 Jun 7;20(19-20):2431-8. doi: 10.1016/s0264-410x(02)00155-x. Vaccine. 2002. PMID: 12057597 Review.
Cited by
-
Immunogenicity of RSV Fusion Protein Adsorbed to Non-Pathogenic Bacillus subtilis Spores: Implications for Mucosal Vaccine Delivery in Nonclinical Animal Models.Biomedicines. 2025 May 3;13(5):1112. doi: 10.3390/biomedicines13051112. Biomedicines. 2025. PMID: 40426939 Free PMC article.
-
The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation.AAPS PharmSciTech. 2020 Aug 5;21(6):225. doi: 10.1208/s12249-020-01744-7. AAPS PharmSciTech. 2020. PMID: 32761294 Free PMC article. Review.
-
Recent advances in respiratory immunization: A focus on COVID-19 vaccines.J Control Release. 2023 Mar;355:655-674. doi: 10.1016/j.jconrel.2023.02.011. Epub 2023 Feb 17. J Control Release. 2023. PMID: 36787821 Free PMC article. Review.
-
Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine.J Control Release. 2018 Dec 28;292:111-118. doi: 10.1016/j.jconrel.2018.10.020. Epub 2018 Oct 16. J Control Release. 2018. PMID: 30339906 Free PMC article.
-
Intranasal influenza-vectored vaccine expressing pneumococcal surface protein A protects against Influenza and Streptococcus pneumoniae infections.NPJ Vaccines. 2024 Dec 19;9(1):246. doi: 10.1038/s41541-024-01033-5. NPJ Vaccines. 2024. PMID: 39702744 Free PMC article.
References
-
- Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411-6. PMID:20466528 - PubMed
-
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. I. Effect on the antibody response to bovine serum albumin and sheep red blood cells of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980;39:426-34. PMID:6248282 - PMC - PubMed
-
- Harris JR, Soliakov A, Lewis RJ, Depoix F, Watkinson A, Lakey JH. Alhydrogel(R) adjuvant, ultrasonic dispersion and protein binding: a TEM and analytical study. Micron. 2012;43:192-200. PMID:21831642 - PubMed
-
- Butler WT, Rossen RD, Wenden RD. Effect of physical state and route of inoculation of diphtheria toxoid on the formation of nasal secretory and serum antibodies in man. J Immunol. 1970;104:1396-400. PMID:5463220 - PubMed
-
- Isaka M, Yasuda Y, Kozuka S, Miura Y, Taniguchi T, Matano K, Goto N, Tochikubo K. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine. 1998;16:1620-6. PMID:9713937 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
