Structure and Catalysis of Fe(III) and Cu(II) Microperoxidase-11 Interacting with the Positively Charged Interfaces of Lipids
- PMID: 28933729
- PMCID: PMC6151982
- DOI: 10.3390/molecules22081212
Structure and Catalysis of Fe(III) and Cu(II) Microperoxidase-11 Interacting with the Positively Charged Interfaces of Lipids
Abstract
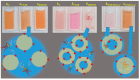
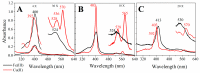
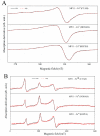
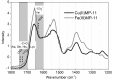
Numerous applications have been described for microperoxidases (MPs) such as in photoreceptors, sensing, drugs, and hydrogen evolution. The last application was obtained by replacing Fe(III), the native central metal, by cobalt ion and inspired part of the present study. Here, the Fe(III) of MP-11 was replaced by Cu(II) that is also a stable redox state in aerated medium, and the structure and activity of both MPs were modulated by the interaction with the positively charged interfaces of lipids. Comparative spectroscopic characterization of Fe(III) and Cu(II)MP-11 in the studied media demonstrated the presence of high and low spin species with axial distortion. The association of the Fe(III)MP-11 with CTAB and Cu(II)MP-11 with DODAB affected the colloidal stability of the surfactants that was recovered by heating. This result is consistent with hydrophobic interactions of MPs with DODAB vesicles and CTAB micelles. The hydrophobic interactions decreased the heme accessibility to substrates and the Fe(III) MP-11catalytic efficiency. Cu(II)MP-11 challenged by peroxides exhibited a cyclic Cu(II)/Cu(I) interconversion mechanism that is suggestive of a mimetic Cu/ZnSOD (superoxide dismutase) activity against peroxides. Hydrogen peroxide-activated Cu(II)MP-11 converted Amplex Red® to dihydroresofurin. This study opens more possibilities for technological applications of MPs.
Keywords: CTAB micelles; DODAB large vesicles; EPR; MCD; microperoxidase-11.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

