Involvement of the Integrin α1β1 in the Progression of Colorectal Cancer
- PMID: 28933766
- PMCID: PMC5575599
- DOI: 10.3390/cancers9080096
Involvement of the Integrin α1β1 in the Progression of Colorectal Cancer
Abstract
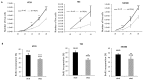
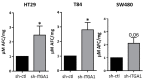
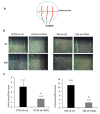
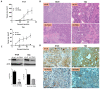
Integrins are a family of heterodimeric glycoproteins involved in bidirectional cell signaling that participate in the regulation of cell shape, adhesion, migration, survival and proliferation. The integrin α1β1 is known to be involved in RAS/ERK proliferative pathway activation and plays an important role in fibroblast proliferation. In the small intestine, the integrin α1 subunit is present in the crypt proliferative compartment and absent in the villus. We have recently shown that the integrin α1 protein and transcript (ITGA1) are present in a large proportion of colorectal cancers (CRC) and that their expression is controlled by the MYC oncogenic factor. Considering that α1 subunit/ITGA1 expression is correlated with MYC in more than 70% of colon adenocarcinomas, we postulated that the integrin α1β1 has a pro-tumoral contribution to CRC. In HT29, T84 and SW480 CRC cells, α1 subunit/ITGA1 knockdown resulted in a reduction of cell proliferation associated with an impaired resistance to anoikis and an altered cell migration in HT29 and T84 cells. Moreover, tumor development in xenografts was reduced in HT29 and T84 sh-ITGA1 cells, associated with extensive necrosis, a low mitotic index and a reduced number of blood vessels. Our results show that α1β1 is involved in tumor cell proliferation, survival and migration. This finding suggests that α1β1 contributes to CRC progression.
Keywords: ITGA1; cell migration; colorectal cancer; integrin α1; proliferation; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Integrin α1β1 expression is controlled by c-MYC in colorectal cancer cells.Oncogene. 2016 Mar 31;35(13):1671-8. doi: 10.1038/onc.2015.231. Epub 2015 Jun 22. Oncogene. 2016. PMID: 26096932 Free PMC article.
-
Integrin α1 subunit is up-regulated in colorectal cancer.Biomark Res. 2013 Mar 7;1(1):16. doi: 10.1186/2050-7771-1-16. Biomark Res. 2013. PMID: 24252313 Free PMC article.
-
Integrin α1 promotes tumorigenicity and progressive capacity of colorectal cancer.Int J Biol Sci. 2020 Jan 16;16(5):815-826. doi: 10.7150/ijbs.37275. eCollection 2020. Int J Biol Sci. 2020. PMID: 32071551 Free PMC article.
-
Integrin α1β1.Adv Exp Med Biol. 2014;819:21-39. doi: 10.1007/978-94-017-9153-3_2. Adv Exp Med Biol. 2014. PMID: 25023165 Review.
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
Cited by
-
The potential prognostic values of the ADAMTS-like protein family: an integrative pan-cancer analysis.Ann Transl Med. 2021 Oct;9(20):1562. doi: 10.21037/atm-21-4946. Ann Transl Med. 2021. PMID: 34790768 Free PMC article.
-
Periostin regulates autophagy through integrin α5β1 or α6β4 and an AKT-dependent pathway in colorectal cancer cell migration.J Cell Mol Med. 2020 Nov;24(21):12421-12432. doi: 10.1111/jcmm.15756. Epub 2020 Sep 29. J Cell Mol Med. 2020. PMID: 32990415 Free PMC article.
-
The characteristics and the multiple functions of integrin β1 in human cancers.J Transl Med. 2023 Nov 6;21(1):787. doi: 10.1186/s12967-023-04696-1. J Transl Med. 2023. PMID: 37932738 Free PMC article. Review.
-
Twist2 is NFkB-responsive when p120-catenin is inactivated and EGFR is overexpressed in esophageal keratinocytes.Sci Rep. 2020 Nov 2;10(1):18829. doi: 10.1038/s41598-020-75866-0. Sci Rep. 2020. PMID: 33139779 Free PMC article.
-
Loss of enteric neuronal Ndrg4 promotes colorectal cancer via increased release of Nid1 and Fbln2.EMBO Rep. 2021 Jun 4;22(6):e51913. doi: 10.15252/embr.202051913. Epub 2021 Apr 23. EMBO Rep. 2021. PMID: 33890711 Free PMC article.
References
-
- Giancotti F.G. Integrin signaling: Specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997;9:691–700. - PubMed
-
- Wary K.K., Mariotti A., Zurzolo C., Giancotti F.G. A requirement for caveolin-1 and associated kinase fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

