A Lanosteryl Triterpene from Protorhus longifolia Improves Glucose Tolerance and Pancreatic Beta Cell Ultrastructure in Type 2 Diabetic Rats
- PMID: 28933769
- PMCID: PMC6152316
- DOI: 10.3390/molecules22081252
A Lanosteryl Triterpene from Protorhus longifolia Improves Glucose Tolerance and Pancreatic Beta Cell Ultrastructure in Type 2 Diabetic Rats
Abstract
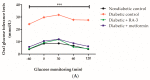
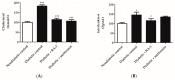
Type 2 diabetes remains one of the leading causes of death worldwide. Persistent hyperglycemia within a diabetic state is implicated in the generation of oxidative stress and aggravated inflammation that is responsible for accelerated modification of pancreatic beta cell structure. Here we investigated whether a lanosteryl triterpene, methyl-3β-hydroxylanosta-9,24-dien-21-oate (RA-3), isolated from Protorhus longifolia can improve glucose tolerance and pancreatic beta cell ultrastructure by reducing oxidative stress and inflammation in high fat diet and streptozotocin-induced type 2 diabetes in rats. In addition to impaired glucose tolerance, the untreated diabetic rats showed increased fasting plasma glucose and C-peptide levels. These untreated diabetic rats further demonstrated raised cholesterol, interleukin-6 (IL-6), and lipid peroxidation levels as well as a destroyed beta cell ultrastructure. Treatment with RA-3 was as effective as metformin in improving glucose tolerance and antioxidant effect in the diabetic rats. Interestingly, RA-3 displayed a slightly more enhanced effect than metformin in reducing elevated IL-6 levels and in improving beta cell ultrastructure. Although the involved molecular mechanisms remain to be established, RA-3 demonstrates a strong potential to improve pancreatic beta cell ultrastructure by attenuating impaired glucose tolerance, reducing oxidative stress and inflammation.
Keywords: Protorhus longifolia; antioxidants; hyperglycemia; hyperlipidemia; inflammation; oxidative stress; pancreatic beta cells; triterpenes; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Figures



Similar articles
-
A Lanosteryl triterpene from Protorhus longifolia augments insulin signaling in type 1 diabetic rats.BMC Complement Altern Med. 2018 Oct 1;18(1):265. doi: 10.1186/s12906-018-2337-z. BMC Complement Altern Med. 2018. PMID: 30285704 Free PMC article.
-
In vivo antihyperglycemic activity of a lanosteryl triterpene from Protorhus longifolia.Molecules. 2015 Jul 22;20(7):13374-83. doi: 10.3390/molecules200713374. Molecules. 2015. PMID: 26205060 Free PMC article.
-
Cardioprotective potential of a lanosteryl triterpene from Protorhus longifolia.Pharm Biol. 2016 Dec;54(12):3244-3248. doi: 10.1080/13880209.2016.1223144. Epub 2016 Aug 30. Pharm Biol. 2016. PMID: 27572517
-
Lanosteryl triterpenes from Protorhus longifolia as a cardioprotective agent: a mini review.Heart Fail Rev. 2019 Jan;24(1):155-166. doi: 10.1007/s10741-018-9733-9. Heart Fail Rev. 2019. PMID: 30167929 Review.
-
Protection of pancreatic beta-cells: is it feasible?Nutr Metab Cardiovasc Dis. 2008 Jan;18(1):74-83. doi: 10.1016/j.numecd.2007.05.004. Nutr Metab Cardiovasc Dis. 2008. PMID: 18096375 Review.
Cited by
-
A Lanosteryl Triterpene (RA-3) Exhibits Antihyperuricemic and Nephroprotective Effects in Rats.Molecules. 2020 Sep 2;25(17):4010. doi: 10.3390/molecules25174010. Molecules. 2020. PMID: 32887389 Free PMC article.
-
A Lanosteryl triterpene from Protorhus longifolia augments insulin signaling in type 1 diabetic rats.BMC Complement Altern Med. 2018 Oct 1;18(1):265. doi: 10.1186/s12906-018-2337-z. BMC Complement Altern Med. 2018. PMID: 30285704 Free PMC article.
-
Two Triterpenoids, ARM-2 and RA-5, From Protorhus longifolia Exhibit the Potential to Modulate Lipolysis and Lipogenesis in Cultured 3T3-L1 Adipocytes.J Lipids. 2024 Oct 17;2024:3972941. doi: 10.1155/2024/3972941. eCollection 2024. J Lipids. 2024. PMID: 39450349 Free PMC article.
-
Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus.Evid Based Complement Alternat Med. 2021 Jul 14;2021:2920530. doi: 10.1155/2021/2920530. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34335803 Free PMC article. Review.
-
Chrysin protects cardiac H9c2 cells against H2O2-induced endoplasmic reticulum stress by up-regulating the Nrf2/PERK pathway.Mol Cell Biochem. 2023 Mar;478(3):539-553. doi: 10.1007/s11010-022-04531-z. Epub 2022 Aug 9. Mol Cell Biochem. 2023. PMID: 35943656
References
-
- International Diabetes Federation (IDF) [(accessed on 29 May 2017)];IDF Diabetes Atlas. (7th ed.). Available online: http://www.diabetesatlas.org/
-
- World Health Organization (WHO) World Health Statistics 2012. [(accessed on 29 May 2017)]; Available online: http://apps.who.int/iris/bitstream/10665/44844/1/9789241564441_eng.pdf?ua=1.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

