Trends in utilization and costs of BRCA testing among women aged 18-64 years in the United States, 2003-2014
- PMID: 28933789
- PMCID: PMC8485755
- DOI: 10.1038/gim.2017.118
Trends in utilization and costs of BRCA testing among women aged 18-64 years in the United States, 2003-2014
Abstract
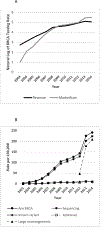
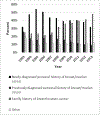
PurposeWe examined 12-year trends in BRCA testing rates and costs in the context of clinical guidelines, national policies, and other factors.MethodsWe estimated trends in BRCA testing rates and costs from 2003 to 2014 for women aged 18-64 years using private claims data and publicly reported revenues from the primary BRCA testing provider.ResultsThe percentage of women with zero out-of-pocket payments for BRCA testing increased during 2013-2014, after 7 years of general decline, coinciding with a clarification of Affordable Care Act coverage of BRCA genetic testing. Beginning in 2007, family history accounted for an increasing proportion of women with BRCA tests compared with personal history, coinciding with BRCA testing guidelines for primary care settings and direct-to-consumer advertising campaigns. During 2013-2014, BRCA testing rates based on claims grew at a faster rate than revenues, following 3 years of similar growth, consistent with increased marketplace competition. In 2013, BRCA testing rates based on claims increased 57%, compared with 11% average annual increases over the preceding 3 years, coinciding with celebrity publicity.ConclusionThe observed trends in BRCA testing rates and costs are consistent with possible effects of several factors, including the Affordable Care Act, clinical guidelines and celebrity publicity.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to disclose.
Figures



References
-
- Moyer VA. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281. - PubMed
-
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Genetic/Familial High Risk Assessment: Breast and Ovarian. 2016. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.AccessedAugust 29, 2016.
-
- U.S. Food and Drug Administration. FDA approves Lynparza to treat advanced ovarian cancer. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427554.htm.AccessedJuly 25, 2016.
-
- Robson ME, Bradbury AR, Arun B, et al.American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol. 2015;33(31):3660–3667. - PubMed
-
- Berliner JL, Fay AM, Cummings SA, Burnett B, Tillmanns T. NSGC practice guideline: risk assessment and genetic counseling for hereditary breast and ovarian cancer. J Genet Couns. 2013;22(2):155–163. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

