Surface Chemistry of Perovskite-Type Electrodes During High Temperature CO2 Electrolysis Investigated by Operando Photoelectron Spectroscopy
- PMID: 28933825
- PMCID: PMC5740481
- DOI: 10.1021/acsami.7b10673
Surface Chemistry of Perovskite-Type Electrodes During High Temperature CO2 Electrolysis Investigated by Operando Photoelectron Spectroscopy
Abstract
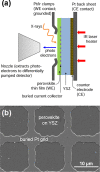
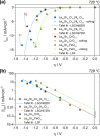
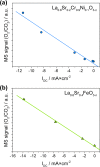
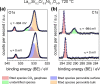
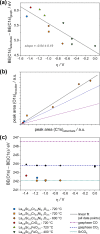
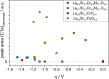
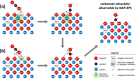
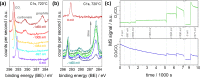
Any substantial move of energy sources from fossil fuels to renewable resources requires large scale storage of excess energy, for example, via power to fuel processes. In this respect electrochemical reduction of CO2 may become very important, since it offers a method of sustainable CO production, which is a crucial prerequisite for synthesis of sustainable fuels. Carbon dioxide reduction in solid oxide electrolysis cells (SOECs) is particularly promising owing to the high operating temperature, which leads to both improved thermodynamics and fast kinetics. Additionally, compared to purely chemical CO formation on oxide catalysts, SOECs have the outstanding advantage that the catalytically active oxygen vacancies are continuously formed at the counter electrode, and move to the working electrode where they reactivate the oxide surface without the need of a preceding chemical (e.g., by H2) or thermal reduction step. In the present work, the surface chemistry of (La,Sr)FeO3-δ and (La,Sr)CrO3-δ based perovskite-type electrodes was studied during electrochemical CO2 reduction by means of near-ambient pressure X-ray photoelectron spectroscopy (NAP-XPS) at SOEC operating temperatures. These measurements revealed the formation of a carbonate intermediate, which develops on the oxide surface only upon cathodic polarization (i.e., under sufficiently reducing conditions). The amount of this adsorbate increases with increasing oxygen vacancy concentration of the electrode material, thus suggesting vacant oxygen lattice sites as the predominant adsorption sites for carbon dioxide. The correlation of carbonate coverage and cathodic polarization indicates that an electron transfer is required to form the carbonate and thus to activate CO2 on the oxide surface. The results also suggest that acceptor doped oxides with high electron concentration and high oxygen vacancy concentration may be particularly suited for CO2 reduction. In contrast to water splitting, the CO2 electrolysis reaction was not significantly affected by metallic particles, which were exsolved from the perovskite electrodes upon cathodic polarization. Carbon formation on the electrode surface was only observed under very strong cathodic conditions, and the carbon could be easily removed by retracting the applied voltage without damaging the electrode, which is particularly promising from an application point of view.
Keywords: CO2 splitting; NAP-XPS; SOEC; defect chemistry; electron transfer; metal exsolution; mixed ionic electronic conductor; solid oxide electrolysis cell.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Krol R. v. d.; Grätzel M. In Photoelectrochemical Hydrogen Production; Tuller H. L., Ed.; Springer: New York, 2012; p 321.
-
- Kurzweil P.HISTORY|Electrochemistry. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, 2009; pp 533–554.
-
- Pöhlmann F.; Jess A. Influence of Syngas Composition on the Kinetics of Fischer–Tropsch Synthesis of using Cobalt as Catalyst. Energy Technol. 2016, 4, 55–64. 10.1002/ente.201500216. - DOI
-
- Graves C.; Ebbesen S. D.; Mogensen M.; Lackner K. S. Sustainable Hydrocarbon Fuels by Recycling CO2 and H2O with Renewable or Nuclear Energy. Renewable Sustainable Energy Rev. 2011, 15, 1–23. 10.1016/j.rser.2010.07.014. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

