Adipogenic Effects and Gene Expression Profiling of Firemaster® 550 Components in Human Primary Preadipocytes
- PMID: 28934090
- PMCID: PMC5915190
- DOI: 10.1289/EHP1318
Adipogenic Effects and Gene Expression Profiling of Firemaster® 550 Components in Human Primary Preadipocytes
Abstract
Background: Exposure to flame retardants has been associated with negative health outcomes including metabolic effects. As polybrominated diphenyl ether flame retardants were pulled from commerce, human exposure to new flame retardants such as Firemaster® 550 (FM550) has increased. Although previous studies in murine systems have shown that FM550 and its main components increase adipogenesis, the effects of FM550 in human models have not been elucidated.
Objectives: The objectives of this study were to determine if FM550 and its components are active in human preadipocytes, and to further investigate their mode of action.
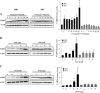
Methods: Human primary preadipocytes were differentiated in the presence of FM550 and its components. Differentiation was assessed by lipid accumulation and expression of peroxisome proliferator-activated receptor γ (PPARG), fatty acid binding protein (FABP) 4 and lipoprotein lipase (LPL). mRNA was collected for Poly (A) RNA sequencing and was used to identify differentially expressed genes (DEGs). Functional analysis of DEGs was undertaken in Ingenuity Pathway Analysis.
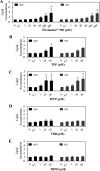
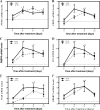
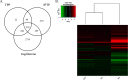
Results: FM550 triphenyl phosphate (TPP) and isopropylated triphenyl phosphates (IPTP), increased adipogenesis in human primary preadipocytes as assessed by lipid accumulation and mRNA expression of regulators of adipogenesis such as PPARγ, CCAAT enhancer binding protein (C/EBP) α and sterol regulatory element binding protein (SREBP) 1 as well as the adipogenic markers FABP4 LPL and perilipin. Poly (A) RNA sequencing analysis revealed potential modes of action including liver X receptor/retinoid X receptor (LXR/RXR) activation, thyroid receptor (TR)/RXR, protein kinase A, and nuclear receptor subfamily 1 group H members activation.
Conclusions: We found that FM550, and two of its components, induced adipogenesis in human primary preadipocytes. Further, using global gene expression analysis we showed that both TPP and IPTP likely exert their effects through PPARG to induce adipogenesis. In addition, IPTP perturbed signaling pathways that were not affected by TPP. https://doi.org/10.1289/EHP1318.
Figures

Similar articles
-
Firemaster® 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Pparγ) on the adipocyte protein 2 (aP2) promoter.PLoS One. 2017 Apr 24;12(4):e0175855. doi: 10.1371/journal.pone.0175855. eCollection 2017. PLoS One. 2017. PMID: 28437481 Free PMC article.
-
Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro.Environ Health Perspect. 2014 Nov;122(11):1225-32. doi: 10.1289/ehp.1408111. Epub 2014 Jul 25. Environ Health Perspect. 2014. PMID: 25062436 Free PMC article.
-
Dechlorane Plus increases adipogenesis in 3T3-L1 and human primary preadipocytes independent of peroxisome proliferator-activated receptor γ transcriptional activity.Int J Obes (Lond). 2019 Mar;43(3):545-555. doi: 10.1038/s41366-018-0072-7. Epub 2018 May 1. Int J Obes (Lond). 2019. PMID: 29717277
-
Adipogenic activity of 2-ethylhexyl diphenyl phosphate via peroxisome proliferator-activated receptor γ pathway.Sci Total Environ. 2020 Apr 1;711:134810. doi: 10.1016/j.scitotenv.2019.134810. Epub 2019 Nov 18. Sci Total Environ. 2020. PMID: 31812418
-
Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation.Int J Obes (Lond). 2016 Oct;40(10):1566-1573. doi: 10.1038/ijo.2016.95. Epub 2016 May 13. Int J Obes (Lond). 2016. PMID: 27273607
Cited by
-
DNA methylation during human adipogenesis and the impact of fructose.Genes Nutr. 2020 Nov 26;15(1):21. doi: 10.1186/s12263-020-00680-2. Genes Nutr. 2020. PMID: 33243154 Free PMC article.
-
A case-control study of exposure to organophosphate flame retardants and risk of thyroid cancer in women.BMC Cancer. 2018 Jun 5;18(1):637. doi: 10.1186/s12885-018-4553-9. BMC Cancer. 2018. PMID: 29871608 Free PMC article.
-
Organophosphate ester flame retardants and plasticizers affect the phenotype and function of HepG2 liver cells.Toxicol Sci. 2024 May 28;199(2):261-275. doi: 10.1093/toxsci/kfae034. Toxicol Sci. 2024. PMID: 38518089 Free PMC article.
-
Skeletal effects following developmental flame-retardant exposure are specific to sex and chemical class in the adult Wistar rat.Front Toxicol. 2023 Jul 27;5:1216388. doi: 10.3389/ftox.2023.1216388. eCollection 2023. Front Toxicol. 2023. PMID: 37577032 Free PMC article.
-
Transgenerational effects of obesogens.Basic Clin Pharmacol Toxicol. 2019 Aug;125 Suppl 3(Suppl 3):44-57. doi: 10.1111/bcpt.13214. Epub 2019 Apr 10. Basic Clin Pharmacol Toxicol. 2019. PMID: 30801972 Free PMC article. Review.
References
-
- Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. 2014. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Lett 228(2):93–102, PMID: 24786373, 10.1016/j.toxlet.2014.04.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

