Invasion of Dendritic Cells, Macrophages and Neutrophils by the Bordetella Adenylate Cyclase Toxin: A Subversive Move to Fool Host Immunity
- PMID: 28934122
- PMCID: PMC5666340
- DOI: 10.3390/toxins9100293
Invasion of Dendritic Cells, Macrophages and Neutrophils by the Bordetella Adenylate Cyclase Toxin: A Subversive Move to Fool Host Immunity
Abstract
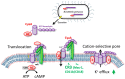
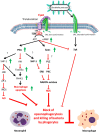
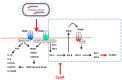
Adenylate cyclase toxin (CyaA) is released in the course of B. pertussis infection in the host's respiratory tract in order to suppress its early innate and subsequent adaptive immune defense. CD11b-expressing dendritic cells (DC), macrophages and neutrophils are professional phagocytes and key players of the innate immune system that provide a first line of defense against invading pathogens. Recent findings revealed the capacity of B. pertussis CyaA to intoxicate DC with high concentrations of 3',5'-cyclic adenosine monophosphate (cAMP), which ultimately skews the host immune response towards the expansion of Th17 cells and regulatory T cells. CyaA-induced cAMP signaling swiftly incapacitates opsonophagocytosis, oxidative burst and NO-mediated killing of bacteria by neutrophils and macrophages. The subversion of host immune responses by CyaA after delivery into DC, macrophages and neutrophils is the subject of this review.
Keywords: T-helper cells; immune response; intracellular pathways; phagocytosis.
Conflict of interest statement
P.S. is a co-inventor of patents protecting the use of CyaA toxoids in vaccines and is founder and share-holder of Revabiotech SE which is developing the next generation of whole cell pertussis vaccines.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

