Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model
- PMID: 28934207
- PMCID: PMC5626502
- DOI: 10.1371/journal.pntd.0005942
Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model
Abstract
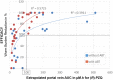
After more than 40 years of use, Praziquantel (PZQ) still remains the drug of choice for the treatment of intestinal and urogenital schistosomiasis. Its anti-parasitic activity resides primarily in the (R)-enantiomer. Hitherto neither the molecular target nor the pharmacokinetic-pharmacodynamic relationship have been fully elucidated. Here we investigated the efficacy and pharmacokinetics of PZQ in the Schistosoma mansoni mouse model to determine the key factors that drive its efficacy. Dose-response studies with racemic PZQ with or without addition of an irreversible pan-cytochrome P450 (CYP) inhibitor, 1-aminobenzotriazole (ABT), were performed. In addition, efficacy of PZQ in the presence of the CYP inducer, dexamethasone (DEX), was determined. Plasma samples were obtained by tail vein bleeding at 4 time points. The (R)-PZQ levels were determined using a LC-MS/MS method. Non-compartmental pharmacokinetic analysis was performed using PKsolver. In addition, experiments using an enhanced in vitro assay were conducted. We found that the use of ABT increased (R)-PZQ plasma exposures in the systemic circulation by ~10 to 20 fold but the latter were not predictive of efficacy. The use of DEX decreased plasma exposures of (R)-PZQ in the systemic circulation by ~10 fold without reducing efficacy. We extrapolated the (R)-PZQ concentrations in mouse portal vein / mesenteric veins from the systemic exposures and found that a free exposure of (R)-PZQ of ~ 20 μM*h in the portal vein was needed to obtain a worm burden reduction >60%. It is suggested that the high (R)-PZQ concentrations available before the hepatic first pass metabolism drive the efficacy against S. mansoni adult worms residing in the mesenteric veins. It is then possible that the current dosing regimen of 40 mg/kg in preventive chemotherapy programs may provide suboptimal concentrations in low-weight patients such as children, due to smaller total amounts of drug administered, and may consequently result in lower cure rates.
Conflict of interest statement
NA is employed by MMV and has received funding by Merck for this work.
Figures

References
-
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014; 383: 2253–64 doi: 10.1016/S0140-6736(13)61949-2 - DOI - PMC - PubMed
-
- Ross AGP, Bartley PB, Sleigh AC et al. Schistosomiasis. N Eng J Med. 2002; 346: 1212–20 - PubMed
-
- Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol. 2016; 46: 395–404 doi: 10.1016/j.ijpara.2016.02.006 - DOI - PubMed
-
- World Health Organization. Schistosomiasis (Fact sheet No. 115); 2013; Retrieved from http://www.who.int/topics/tropical_diseases/factsheets/neglected/en/. Accessed on 21st of October 2016.
-
- Bustinduy AL, Wright S, Joekes EC et al. One hundred years of neglect in paediatric schistosomiasis. Parasitology 2017; ahead of print doi: 10.1017/S0031182017000014 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

