Ursodeoxycholic acid prevents ventricular conduction slowing and arrhythmia by restoring T-type calcium current in fetuses during cholestasis
- PMID: 28934223
- PMCID: PMC5608194
- DOI: 10.1371/journal.pone.0183167
Ursodeoxycholic acid prevents ventricular conduction slowing and arrhythmia by restoring T-type calcium current in fetuses during cholestasis
Abstract
Background: Increased maternal serum bile acid concentrations in intrahepatic cholestasis of pregnancy (ICP) are associated with fetal cardiac arrhythmias. Ursodeoxycholic acid (UDCA) has been shown to demonstrate anti-arrhythmic properties via preventing ICP-associated cardiac conduction slowing and development of reentrant arrhythmias, although the cellular mechanism is still being elucidated.
Methods: High-resolution fluorescent optical mapping of electrical activity and electrocardiogram measurements were used to characterize effects of UDCA on one-day-old neonatal and adult female Langendorff-perfused rat hearts. ICP was modelled by perfusion of taurocholic acid (TC, 400μM). Whole-cell calcium currents were recorded from neonatal rat and human fetal cardiomyocytes.
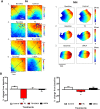
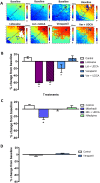
Results: TC significantly prolonged the PR interval by 11.0±3.5% (P<0.05) and slowed ventricular conduction velocity (CV) by 38.9±5.1% (P<0.05) exclusively in neonatal and not in maternal hearts. A similar CV decline was observed with the selective T-type calcium current (ICa,T) blocker mibefradil 1μM (23.0±6.2%, P<0.05), but not with the L-type calcium current (ICa,L) blocker nifedipine 1μM (6.9±6.6%, NS). The sodium channel blocker lidocaine (30μM) reduced CV by 60.4±4.5% (P<0.05). UDCA co-treatment was protective against CV slowing induced by TC and mibefradil, but not against lidocaine. UDCA prevented the TC-induced reduction in the ICa,T density in both isolated human fetal (-10.2±1.5 versus -5.5±0.9 pA/pF, P<0.05) and neonatal rat ventricular myocytes (-22.3±1.1 versus -9.6±0.8 pA/pF, P<0.0001), whereas UDCA had limited efficacy on the ICa,L.
Conclusion: Our findings demonstrate that ICa,T plays a significant role in ICP-associated fetal cardiac conduction slowing and arrhythmogenesis, and is an important component of the fetus-specific anti-arrhythmic activity of UDCA.
Conflict of interest statement
Figures



Similar articles
-
Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations.J Hepatol. 2021 May;74(5):1087-1096. doi: 10.1016/j.jhep.2020.11.038. Epub 2020 Dec 1. J Hepatol. 2021. PMID: 33276032 Free PMC article.
-
The protective effect of ursodeoxycholic acid in an in vitro model of the human fetal heart occurs via targeting cardiac fibroblasts.Prog Biophys Mol Biol. 2016 Jan;120(1-3):149-63. doi: 10.1016/j.pbiomolbio.2016.01.003. Epub 2016 Jan 8. Prog Biophys Mol Biol. 2016. PMID: 26777584
-
A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart.Hepatology. 2011 Oct;54(4):1282-92. doi: 10.1002/hep.24492. Epub 2011 Aug 1. Hepatology. 2011. PMID: 21809354 Free PMC article.
-
Bile acid signaling in fetal tissues: implications for intrahepatic cholestasis of pregnancy.Dig Dis. 2011;29(1):58-61. doi: 10.1159/000324130. Epub 2011 Jun 17. Dig Dis. 2011. PMID: 21691106 Review.
-
Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications.Clin Sci (Lond). 2011 Dec;121(12):523-44. doi: 10.1042/CS20110184. Clin Sci (Lond). 2011. PMID: 21854363 Review.
Cited by
-
Molecular Pathogenesis of Intrahepatic Cholestasis of Pregnancy.Can J Gastroenterol Hepatol. 2021 May 30;2021:6679322. doi: 10.1155/2021/6679322. eCollection 2021. Can J Gastroenterol Hepatol. 2021. PMID: 34195157 Free PMC article. Review.
-
Intrahepatic cholestasis of pregnancy.Nat Rev Dis Primers. 2025 Jul 24;11(1):51. doi: 10.1038/s41572-025-00633-2. Nat Rev Dis Primers. 2025. PMID: 40707479 Review.
-
Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations.J Hepatol. 2021 May;74(5):1087-1096. doi: 10.1016/j.jhep.2020.11.038. Epub 2020 Dec 1. J Hepatol. 2021. PMID: 33276032 Free PMC article.
-
Enzymatic Synthesis of New Acetoacetate-Ursodeoxycholic Acid Hybrids as Potential Therapeutic Agents and Useful Synthetic Scaffolds as Well.Molecules. 2024 Mar 15;29(6):1305. doi: 10.3390/molecules29061305. Molecules. 2024. PMID: 38542941 Free PMC article.
-
The Role of Bile Acids in Cardiovascular Diseases: from Mechanisms to Clinical Implications.Aging Dis. 2023 Apr 1;14(2):261-282. doi: 10.14336/AD.2022.0817. eCollection 2023 Apr 1. Aging Dis. 2023. PMID: 37008052 Free PMC article.
References
-
- Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology 2014;59:1482–1491. doi: 10.1002/hep.26617 - DOI - PMC - PubMed
-
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol 2014;124:120–133. doi: 10.1097/AOG.0000000000000346 - DOI - PubMed
-
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology 2004;40:467–474. doi: 10.1002/hep.20336 - DOI - PubMed
-
- Geenes V, Lovgren-Sandblom A, Benthin L, Lawrance D, Chambers J, Gurung V et al. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid. PLoS One 2014;9:e83828 doi: 10.1371/journal.pone.0083828 - DOI - PMC - PubMed
-
- Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology 2012;143:1492–1501. doi: 10.1053/j.gastro.2012.08.004 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

