Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury
- PMID: 28934227
- PMCID: PMC5608216
- DOI: 10.1371/journal.pone.0184393
Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury
Abstract
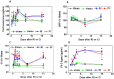
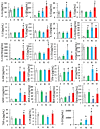
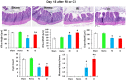
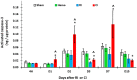
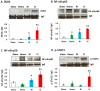
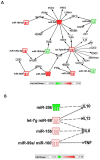
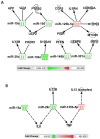
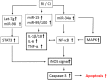
Hemorrhage following whole-body γ-irradiation in a combined injury (CI) model increases mortality compared to whole-body γ-irradiation alone (RI). The decreased survival in CI is accompanied by increased bone marrow injury, decreased hematocrit, and alterations of miRNA in the kidney. In this study, our aim was to examine cytokine homeostasis, susceptibility to systemic bacterial infection, and intestinal injury. More specifically, we evaluated the interleukin-6 (IL-6)-induced stress proteins including C-reactive protein (CRP), complement 3 (C3), Flt-3 ligand, and corticosterone. CD2F1 male mice received 8.75 Gy 60Co gamma photons (0.6 Gy/min, bilateral) which was followed by a hemorrhage of 20% of the blood volume. In serum, RI caused an increase of IL-1, IL-2, IL-3, IL-5, IL-6, IL-12, IL-13, IL-15, IL-17A, IL-18, G-CSF, CM-CSF, eotaxin, IFN-γ, MCP-1, MIP, RANTES, and TNF-α, which were all increased by hemorrhage alone, except IL-9, IL-17A, and MCP-1. Nevertheless, CI further elevated RI-induced increases of these cytokines except for G-CSF, IFN- γ and RANTES in serum. In the ileum, hemorrhage in the CI model significantly enhanced RI-induced IL-1β, IL-3, IL-6, IL-10, IL-12p70, IL-13, IL-18, and TNF-α concentrations. In addition, Proteus mirabilis Gram(-) was found in only 1 of 6 surviving RI mice on Day 15, whereas Streptococcus sanguinis Gram(+) and Sphingomonas paucimobilis Gram(-) were detected in 2 of 3 surviving CI mice (with 3 CI mice diseased due to inflammation and infection before day 15) at the same time point. Hemorrhage in the CI model enhanced the RI-induced increases in C3 and decreases in CRP concentrations. However, hemorrhage alone did not alter the basal levels, but hemorrhage in the CI model displayed similar increases in Flt-3 ligand levels as RI did. Hemorrhage alone altered the basal levels of corticosterone early after injury, which then returned to the baseline, but in RI mice and CI mice the increased corticosterone concentration remained elevated throughout the 15 day study. CI increased 8 miRNAs and decreased 10 miRNAs in serum, and increased 16 miRNA and decreased 6 miRNAs in ileum tissue. Among the altered miRNAs, CI increased miR-34 in the serum and ileum which targeted an increased phosphorylation of ERK, p38, and increased NF-κB, thereby leading to increased iNOS expression and activation of caspase-3 in the ileum. Further, let-7g/miR-98 targeted the increased phosphorylation of STAT3 in the ileum, which is known to bind to the iNOS gene. These changes may correlate with cell death in the ileum of CI mice. The histopathology displayed blunted villi and villus edema in RI and CI mice. Based on the in silico analysis, miR-15, miR-99, and miR-100 were predicted to regulate IL-6 and TNF. These results suggest that CI-induced alterations of cytokines/chemokines, CRP, and C3 cause a homeostatic imbalance and may contribute to the pathophysiology of the gastrointestinal injury. Inhibitory intervention in these responses may prove therapeutic for CI and improve recovery of the ileal morphologic damage.
Conflict of interest statement
Figures


Similar articles
-
Hemorrhage Exacerbates Radiation Effects on Survival, Leukocytopenia, Thrombopenia, Erythropenia, Bone Marrow Cell Depletion and Hematopoiesis, and Inflammation-Associated microRNAs Expression in Kidney.PLoS One. 2015 Sep 30;10(9):e0139271. doi: 10.1371/journal.pone.0139271. eCollection 2015. PLoS One. 2015. PMID: 26422254 Free PMC article.
-
Female Mice are More Resistant to the Mixed-Field (67% Neutron + 33% Gamma) Radiation-Induced Injury in Bone Marrow and Small Intestine than Male Mice due to Sustained Increases in G-CSF and the Bcl-2/Bax Ratio and Lower miR-34a and MAPK Activation.Radiat Res. 2022 Aug 1;198(2):120-133. doi: 10.1667/RADE-21-00201.1. Radiat Res. 2022. PMID: 35452510 Free PMC article.
-
A Combined Therapy of Pegylated G-CSF with Ciprofloxacin Mitigates Damage Induced by Lethal Ionizing Radiation to the Bone Marrow, Spleen, and Ileum by Increasing AKT Activation but Decreasing IL-18, C3, and miR-34a.Radiat Res. 2025 May 1;203(5):341-356. doi: 10.1667/RADE-24-00266.1. Radiat Res. 2025. PMID: 40181563
-
Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E 2 after whole-body reactor-produced mixed field (n + γ-photons) irradiation.Oxid Med Cell Longev. 2013;2013:821541. doi: 10.1155/2013/821541. Epub 2013 Sep 18. Oxid Med Cell Longev. 2013. PMID: 24175013 Free PMC article.
-
Systemic inflammatory response to exhaustive exercise. Cytokine kinetics.Exerc Immunol Rev. 2002;8:6-48. Exerc Immunol Rev. 2002. PMID: 12690937 Review.
Cited by
-
A clinically-relevant mouse model that displays hemorrhage exacerbates tourniquet-induced acute kidney injury.Front Physiol. 2023 Nov 8;14:1240352. doi: 10.3389/fphys.2023.1240352. eCollection 2023. Front Physiol. 2023. PMID: 38028812 Free PMC article.
-
Circulating Cytokine/Chemokine Concentrations Respond to Ionizing Radiation Doses but not Radiation Dose Rates: Granulocyte-Colony Stimulating Factor and Interleukin-18.Radiat Res. 2018 Jun;189(6):634-643. doi: 10.1667/RR14966.1. Epub 2018 Apr 13. Radiat Res. 2018. PMID: 29652619 Free PMC article.
-
Early Life Irradiation-Induced Hypoplasia and Impairment of Neurogenesis in the Dentate Gyrus and Adult Depression Are Mediated by MicroRNA- 34a-5p/T-Cell Intracytoplasmic Antigen-1 Pathway.Cells. 2021 Sep 18;10(9):2476. doi: 10.3390/cells10092476. Cells. 2021. PMID: 34572124 Free PMC article.
-
Turning anecdotal irradiation-induced anticancer immune responses into reproducible in situ cancer vaccines via disulfiram/copper-mediated enhanced immunogenic cell death of breast cancer cells.Cell Death Dis. 2024 Apr 27;15(4):298. doi: 10.1038/s41419-024-06644-3. Cell Death Dis. 2024. PMID: 38678042 Free PMC article.
-
Effects of Hemorrhage on Hematopoietic Cell Depletion after a Combined Injury with Radiation: Role of White Blood Cells and Red Blood Cells as Biomarkers.Int J Mol Sci. 2024 Mar 4;25(5):2988. doi: 10.3390/ijms25052988. Int J Mol Sci. 2024. PMID: 38474235 Free PMC article.
References
-
- Kishi HS. Effects of the “special bomb”: recollection of a neurosurgeon in Hiroshima, August 8–15, 1945. Neurosurgery. 2000; 47(2): 441–446. . - PubMed
-
- Iijima S. Pathology of atomic bomb casualties. Acta Pathology Japan. 1982; 32 (Suppl 2): 237–270. . - PubMed
-
- Barabanova AV. Significance of beta-radiation skin burns in Chernobyl patients for the theory and practice of radiopathology. Vojnosanit Pregl. 2006; 63: 477–480. . - PubMed
-
- Ledney GD, Exum ED, Sheehy PA. Survival enhanced by skin-wound trauma in mice exposed to 60Co radiation. Experientia. 1981; 37(2): 193–194. . - PubMed
-
- Ledney GD, Gelston HM Jr, Weinberg SR, Exum ED. Survival and endogenous spleen colonies of irradiated mice after skin wounding and hydroxyurea treatment. Experientia. 1982; 38(10): 1228–1230. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

