Study of myelin structure changes during the nerve fibers demyelination
- PMID: 28934355
- PMCID: PMC5608327
- DOI: 10.1371/journal.pone.0185170
Study of myelin structure changes during the nerve fibers demyelination
Abstract
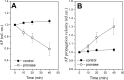
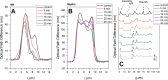
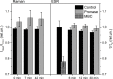
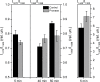
Raman, NMR and EPR spectroscopy and electrophysiology methods were used to investigate the excitability and the packaging of myelin lipid layers and its viscosity during nerve exposure to pronase E. It was established that during exposure of nerve to pronase E the action potential (AP) conduction velocity and the Schwann cell (SC) (or myelin) water ordering increases, but the nerve myelin refractive index and internode incisions numbers decrease. This effect included two periods-short- and long-time period, probably, because the first one depends on SC protein changes and the second one-on the nerve fiber internode demyelination. It was concluded that high electrical resistance of myelin, which is important for a series of AP conduction velocity, not only depends on nerve fiber diameter and the myelin lipid composition, but also on the regularity of myelin lipid fatty acids and myelin lipid layer packing during the axoglial interaction.
Conflict of interest statement
Figures



References
-
- Waxman SG, Kocsis JD, Stys PK. The Axon. Structure, function and pathophysiology Oxford Univ. Press; NY-Oxford: 1995; p.325
-
- Chiu SY, Kriegler S. Neurotransmitter-mediated signaling between axons and glial cells. Glia. 1994; 11:191–200 doi: 10.1002/glia.440110213 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

