Loss of SLC25A46 causes neurodegeneration by affecting mitochondrial dynamics and energy production in mice
- PMID: 28934388
- PMCID: PMC6074941
- DOI: 10.1093/hmg/ddx262
Loss of SLC25A46 causes neurodegeneration by affecting mitochondrial dynamics and energy production in mice
Abstract
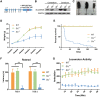
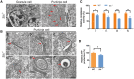
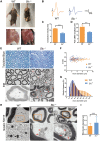
Recently, we identified biallelic mutations of SLC25A46 in patients with multiple neuropathies. Functional studies revealed that SLC25A46 may play an important role in mitochondrial dynamics by mediating mitochondrial fission. However, the cellular basis and pathogenic mechanism of the SLC25A46-related neuropathies are not fully understood. Thus, we generated a Slc25a46 knock-out mouse model. Mice lacking SLC25A46 displayed severe ataxia, mainly caused by degeneration of Purkinje cells. Increased numbers of small, unmyelinated and degenerated optic nerves as well as loss of retinal ganglion cells indicated optic atrophy. Compound muscle action potentials in peripheral nerves showed peripheral neuropathy associated with degeneration and demyelination in axons. Mutant cerebellar neurons have large mitochondria, which exhibit abnormal distribution and transport. Biochemically mutant mice showed impaired electron transport chain activity and accumulated autophagy markers. Our results suggest that loss of SLC25A46 causes degeneration in neurons by affecting mitochondrial dynamics and energy production.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Charlesworth G., Balint B., Mencacci N.E., Carr L., Wood N.W., Bhatia K.P. (2016) SLC25A46 mutations underlie progressive myoclonic ataxia with optic atrophy and neuropathy. Mov. Disord., 31, 1249–1251. - PubMed
-
- Janer A., Prudent J., Paupe V., Fahiminiya S., Majewski J., Sgarioto N., Des Rosiers C., Forest A., Lin Z.Y., Gingras A.C.. et al. (2016) SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol. Med., 8, 1019–1038. - PMC - PubMed
-
- Nguyen M., Boesten I., Hellebrekers D.M., Mulder-den Hartog N.M., de Coo I.F., Smeets H.J., Gerards M. (2017) Novel pathogenic SLC25A46 splice-site mutation causes an optic atrophy spectrum disorder. Clin. Genet., 91, 121–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

