High-Sensitivity Assays for Plasmodium falciparum Infection by Immuno-Polymerase Chain Reaction Detection of PfIDEh and PfLDH Antigens
- PMID: 28934434
- PMCID: PMC5854022
- DOI: 10.1093/infdis/jix369
High-Sensitivity Assays for Plasmodium falciparum Infection by Immuno-Polymerase Chain Reaction Detection of PfIDEh and PfLDH Antigens
Abstract
Background: Rapid diagnostic tests based on Plasmodium falciparum histidine-rich protein II (PfHRP-II) and P. falciparum lactate dehydrogenase (PfLDH) antigens are widely deployed for detection of P. falciparum infection; however, these tests often miss cases of low-level parasitemia, and PfHRP-II tests can give false-negative results when P. falciparum strains do not express this antigen.
Methods: We screened proteomic data for highly expressed P. falciparum proteins and compared their features to those of PfHRP-II and PfLDH biomarkers. Search criteria included high levels of expression, conservation in all parasite strains, and good correlation of antigen levels with parasitemia and its clearance after drug treatment. Different assay methods were compared for sensitive detection of parasitemia in P. falciparum cultures.
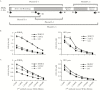
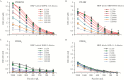
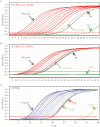
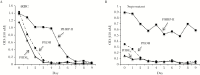
Results: Among potential new biomarkers, a P. falciparum homolog of insulin-degrading enzyme (PfIDEh) met our search criteria. Comparative enzyme-linked immunosorbent assays with monoclonal antibodies against PfLDH or PfIDEh showed detection limits of 100-200 parasites/µL and 200-400 parasites/µL, respectively. Detection was dramatically improved by use of real-time immuno-polymerase chain reaction (PCR), to parasitemia limits of 0.02 parasite/µL and 0.78 parasite/µL in PfLDH- and PfIDEh-based assays, respectively.
Conclusions: The ability of PfLDH- or PfIDEh-based immuno-PCR assays to detect <1 parasite/µL suggests that improvements of bound antibody sensor technology may greatly increase the sensitivity of malaria rapid diagnostic tests.
Keywords: PfHRP-II; enzyme-linked immunosorbent assay; immuno-PCR; insulin-degrading enzyme; lactate dehydrogenase enzyme.
Published by Oxford University Press for the Infectious Diseases Society of America 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
References
-
- World Health Organization Global Malaria Programme. World malaria report. Available at: http://www.who.int/malaria/publications/world-malaria-report-2016/en/. Accessed 24 August 2017.
-
- Slater HC, Ross A, Ouédraogo AL et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 2015; 528:S94–101. - PubMed
-
- Wu L, van den Hoogen LL, Slater H et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015; 528:S86–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

