The important conformational plasticity of DsrA sRNA for adapting multiple target regulation
- PMID: 28934467
- PMCID: PMC5766208
- DOI: 10.1093/nar/gkx570
The important conformational plasticity of DsrA sRNA for adapting multiple target regulation
Abstract
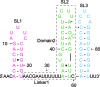
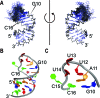
In bacteria, small non-coding RNAs (sRNAs) could function in gene regulations under variable stress responses. DsrA is an ∼90-nucleotide Hfq-dependent sRNA found in Escherichia coli. It regulates the translation and degradation of multiple mRNAs, such as rpoS, hns, mreB and rbsD mRNAs. However, its functional structure and particularly how it regulates multiple mRNAs remain obscure. Using NMR, we investigated the solution structures of the full-length and isolated stem-loops of DsrA. We first solved the NMR structure of the first stem-loop (SL1), and further studied the melting process of the SL1 induced by the base-pairing with the rpoS mRNA and the A-form duplex formation of the DsrA/rpoS complex. The secondary structure of the second stem-loop (SL2) was also determined, which contains a lower stem and an upper stem with distinctive stability. Interestingly, two conformational states of SL2 in dynamic equilibrium were observed in our NMR spectra, suggesting that the conformational selection may occur during the base-pairing between DsrA and mRNAs. In summary, our study suggests that the conformational plasticity of DsrA may represent a special mechanism sRNA employed to deal with its multiple regulatory targets of mRNA.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

