Crystal structure of an engineered, HIV-specific recombinase for removal of integrated proviral DNA
- PMID: 28934476
- PMCID: PMC5766204
- DOI: 10.1093/nar/gkx603
Crystal structure of an engineered, HIV-specific recombinase for removal of integrated proviral DNA
Abstract
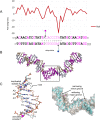
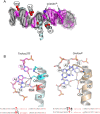
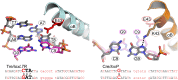
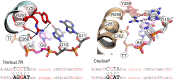
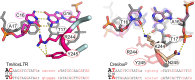
As part of the HIV infection cycle, viral DNA inserts into the genome of host cells such that the integrated DNA encoding the viral proteins is flanked by long terminal repeat (LTR) regions from the retrovirus. In an effort to develop novel genome editing techniques that safely excise HIV provirus from cells, Tre, an engineered version of Cre recombinase, was designed to target a 34-bp sequence within the HIV-1 LTR (loxLTR). The sequence targeted by Tre lacks the symmetry present in loxP, the natural DNA substrate for Cre. We report here the crystal structure of a catalytically inactive (Y324F) mutant of this engineered Tre recombinase in complex with the loxLTR DNA substrate. We also report that 17 of the 19 amino acid changes relative to Cre contribute to the altered specificity, even though many of these residues do not contact the DNA directly. We hypothesize that some mutations increase the flexibility of the Cre tetramer and that this, along with flexibility in the DNA, enable the engineered enzyme and DNA substrate to adopt complementary conformations.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
A novel mutant loxP containing part of long terminal repeat of HIV-1 in spacer region: presentation of possible target site for antiviral strategy using site-specific recombinase.Biochem Biophys Res Commun. 1998 Dec 30;253(3):588-93. doi: 10.1006/bbrc.1998.9814. Biochem Biophys Res Commun. 1998. PMID: 9918772
-
An engineered lox sequence containing part of a long terminal repeat of HIV-1 permits Cre recombinase-mediated DNA excision.Biochem Cell Biol. 2000;78(6):653-8. Biochem Cell Biol. 2000. PMID: 11206575
-
HIV-1 proviral DNA excision using an evolved recombinase.Science. 2007 Jun 29;316(5833):1912-5. doi: 10.1126/science.1141453. Science. 2007. PMID: 17600219
-
Retroviral integrase proteins and HIV-1 DNA integration.J Biol Chem. 2012 Nov 30;287(49):40858-66. doi: 10.1074/jbc.R112.397760. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043109 Free PMC article. Review.
-
In vitro evolution and analysis of HIV-1 LTR-specific recombinases.Methods. 2011 Jan;53(1):102-9. doi: 10.1016/j.ymeth.2010.06.014. Epub 2010 Jun 26. Methods. 2011. PMID: 20600935 Review.
Cited by
-
Protein and DNA Conformational Changes Contribute to Specificity of Cre Recombinase.bioRxiv [Preprint]. 2024 Dec 11:2024.12.11.627928. doi: 10.1101/2024.12.11.627928. bioRxiv. 2024. Update in: Biochemistry. 2025 Mar 04;64(5):1055-1064. doi: 10.1021/acs.biochem.4c00841. PMID: 39713331 Free PMC article. Updated. Preprint.
-
Engineering altered protein-DNA recognition specificity.Nucleic Acids Res. 2018 Jun 1;46(10):4845-4871. doi: 10.1093/nar/gky289. Nucleic Acids Res. 2018. PMID: 29718463 Free PMC article. Review.
-
The recombination efficiency of the bacterial integron depends on the mechanical stability of the synaptic complex.Sci Adv. 2024 Dec 13;10(50):eadp8756. doi: 10.1126/sciadv.adp8756. Epub 2024 Dec 13. Sci Adv. 2024. PMID: 39671485 Free PMC article.
-
Mechanisms of Cre recombinase synaptic complex assembly and activation illuminated by Cryo-EM.Nucleic Acids Res. 2022 Feb 22;50(3):1753-1769. doi: 10.1093/nar/gkac032. Nucleic Acids Res. 2022. PMID: 35104890 Free PMC article.
-
A heterodimer of evolved designer-recombinases precisely excises a human genomic DNA locus.Nucleic Acids Res. 2020 Jan 10;48(1):472-485. doi: 10.1093/nar/gkz1078. Nucleic Acids Res. 2020. PMID: 31745551 Free PMC article.
References
-
- Rathbun R.C., Lockhart S.M., Stephens J.R.. Current HIV treatment guidelines–an overview. Curr. Pharm. Des. 2006; 12:1045–1063. - PubMed
-
- Buchholz F., Hauber J.. Antiviral therapy of persistent viral infection using genome editing. Curr. Opin. Virol. 2016; 20:85–91. - PubMed
-
- van Lunzen J., Fehse B., Hauber J.. Gene therapy strategies: can we eradicate HIV. Curr. HIV/AIDS Rep. 2011; 8:78–84. - PubMed
-
- Govindan G., Ramalingam S.. Programmable site-specific nucleases for targeted genome engineering in higher eukaryotes. J. Cell. Physiol. 2016; 231:2380–2392. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

