Activities of gyrase and topoisomerase IV on positively supercoiled DNA
- PMID: 28934496
- PMCID: PMC5766186
- DOI: 10.1093/nar/gkx649
Activities of gyrase and topoisomerase IV on positively supercoiled DNA
Abstract
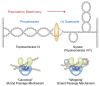
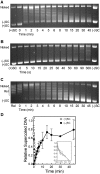
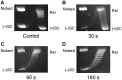
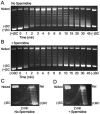
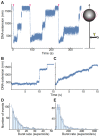
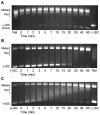
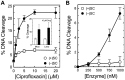
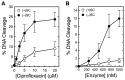
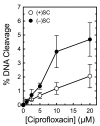
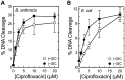
Although bacterial gyrase and topoisomerase IV have critical interactions with positively supercoiled DNA, little is known about the actions of these enzymes on overwound substrates. Therefore, the abilities of Bacillus anthracis and Escherichia coli gyrase and topoisomerase IV to relax and cleave positively supercoiled DNA were analyzed. Gyrase removed positive supercoils ∼10-fold more rapidly and more processively than it introduced negative supercoils into relaxed DNA. In time-resolved single-molecule measurements, gyrase relaxed overwound DNA with burst rates of ∼100 supercoils per second (average burst size was 6.2 supercoils). Efficient positive supercoil removal required the GyrA-box, which is necessary for DNA wrapping. Topoisomerase IV also was able to distinguish DNA geometry during strand passage and relaxed positively supercoiled substrates ∼3-fold faster than negatively supercoiled molecules. Gyrase maintained lower levels of cleavage complexes with positively supercoiled (compared with negatively supercoiled) DNA, whereas topoisomerase IV generated similar levels with both substrates. Results indicate that gyrase is better suited than topoisomerase IV to safely remove positive supercoils that accumulate ahead of replication forks. They also suggest that the wrapping mechanism of gyrase may have evolved to promote rapid removal of positive supercoils, rather than induction of negative supercoils.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Recognition of DNA Supercoil Geometry by Mycobacterium tuberculosis Gyrase.Biochemistry. 2017 Oct 10;56(40):5440-5448. doi: 10.1021/acs.biochem.7b00681. Epub 2017 Sep 25. Biochemistry. 2017. PMID: 28921956 Free PMC article.
-
The mechanism of negative DNA supercoiling: a cascade of DNA-induced conformational changes prepares gyrase for strand passage.DNA Repair (Amst). 2014 Apr;16:23-34. doi: 10.1016/j.dnarep.2014.01.011. Epub 2014 Feb 22. DNA Repair (Amst). 2014. PMID: 24674625 Review.
-
DNA Supercoiling Catalyzed by Bacterial Gyrase.Methods Mol Biol. 2025;2928:39-50. doi: 10.1007/978-1-0716-4550-5_4. Methods Mol Biol. 2025. PMID: 40372635 Free PMC article.
-
Preferential relaxation of positively supercoiled DNA by E. coli topoisomerase IV in single-molecule and ensemble measurements.Genes Dev. 2000 Nov 15;14(22):2881-92. doi: 10.1101/gad.838900. Genes Dev. 2000. PMID: 11090135 Free PMC article.
-
What makes a type IIA topoisomerase a gyrase or a Topo IV?Nucleic Acids Res. 2021 Jun 21;49(11):6027-6042. doi: 10.1093/nar/gkab270. Nucleic Acids Res. 2021. PMID: 33905522 Free PMC article. Review.
Cited by
-
Highly sensitive mapping of in vitro type II topoisomerase DNA cleavage sites with SHAN-seq.bioRxiv [Preprint]. 2024 May 17:2024.05.17.594727. doi: 10.1101/2024.05.17.594727. bioRxiv. 2024. Update in: Nucleic Acids Res. 2024 Sep 9;52(16):9777-9787. doi: 10.1093/nar/gkae638. PMID: 38798569 Free PMC article. Updated. Preprint.
-
Interactions between Zoliflodacin and Neisseria gonorrhoeae Gyrase and Topoisomerase IV: Enzymological Basis for Cellular Targeting.ACS Infect Dis. 2024 Aug 9;10(8):3071-3082. doi: 10.1021/acsinfecdis.4c00438. Epub 2024 Jul 31. ACS Infect Dis. 2024. PMID: 39082980 Free PMC article.
-
Telling Your Right Hand from Your Left: The Effects of DNA Supercoil Handedness on the Actions of Type II Topoisomerases.Int J Mol Sci. 2023 Jul 7;24(13):11199. doi: 10.3390/ijms241311199. Int J Mol Sci. 2023. PMID: 37446377 Free PMC article. Review.
-
Assessing in vivo the impact of gene context on transcription through DNA supercoiling.Nucleic Acids Res. 2023 Oct 13;51(18):9509-9521. doi: 10.1093/nar/gkad688. Nucleic Acids Res. 2023. PMID: 37667073 Free PMC article.
-
Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance.ACS Infect Dis. 2024 Apr 12;10(4):1097-1115. doi: 10.1021/acsinfecdis.4c00128. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38564341 Free PMC article. Review.
References
-
- Wang J.C. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 2002; 3:430–440. - PubMed
-
- Bates A.D., Maxwell A.. DNA Topology. 2005; NY: Oxford University Press.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

