Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer
- PMID: 28934761
- PMCID: PMC5680467
- DOI: 10.1038/bjc.2017.320
Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer
Abstract
Background: Limited data exist regarding the correlation between MRI tumour regression grade (mrTRG) and pathological TRG (pTRG) in rectal cancer.
Methods: mrTRG and pTRG were compared in rectal cancer patients from two phase II trials (EXPERT and EXPERT-C). The agreement between radiologist and pathologist was assessed with the weighted κ test while the Kaplan-Meier method was used to estimate survival outcomes.
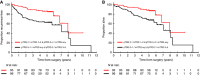
Results: One hundred ninety-one patients were included. Median time from completion of neoadjuvant treatment to pre-operative MRI and surgery was 4.1 weeks (interquartile range (IQR): 3.7-4.7) and 6.6 weeks (IQR: 5.9-7.6), respectively. Fair agreement was found between mrTRG and pTRG when regression was classified according to standard five-tier systems (κ=0.24) or modified three-tier systems (κ=0.25). Sensitivity and specificity of mrTRG 1-2 (complete/good radiological regression) for the prediction of pathological complete response was 74.4% (95% CI: 58.8-86.5) and 62.8% (95% CI: 54.5-70.6), respectively. Survival outcomes of patients with intermediate pathological regression (pTRG 2) were numerically better if complete/good regression was also observed on imaging (mrTRG 1-2) compared to poor regression (mrTRG 3-5) (5-year recurrence-free survival 76.9% vs 65.9%, P=0.18; 5-year overall survival 80.6% vs 68.8%, P=0.22).
Conclusions: The agreement between mrTRG and pTRG is low and mrTRG cannot be used as a surrogate of pTRG. Further studies are warranted to assess the ability of mrTRG to identify pathological complete responders for the adoption of non-operative management strategies and to provide complementary prognostic information to pTRG for better risk-stratification after surgery.
Conflict of interest statement
DC received research funding from: Roche, Amgen, Celgene, Sanofi, Merck Serono, Novartis, AstraZeneca, Bayer, Merrimack and MedImmune. CP has had advisory roles with Sanofi. JT has had advisory roles with Amgen, Roche, Sanofi-Aventis, and Merck. IC has had advisory roles with Merck Serono, Roche, Sanofi Oncology, Bristol Myers Squibb, Eli-Lilly, Novartis, Gilead Science. He has received research funding from Merck-Serono, Novartis, Roche and Sanofi Oncology, and honoraria from Roche, Sanofi-Oncology, Eli-Lilly, Taiho. All other authors declare that they have no conflicts of interest.
Figures
References
-
- Magnetic Resonance Tumour Regression Grade as Biomarker for Stratified Management of Rectal Cancer Patients (TRIGGER). Available at https://clinicaltrials.gov/ct2/show/NCT02704520.
-
- Beets-Tan RG, Beets GL (2004) Rectal cancer: review with emphasis on MR imaging. Radiology 232(2): 335–346. - PubMed
-
- Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, von Meyenfeldt MF, Baeten CG, van Engelshoven JM (2001) Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 357(9255): 497–504. - PubMed
-
- Bhoday J, Smith F, Siddiqui MR, Balyasnikova S, Swift RI, Perez R, Habr-Gama A, Brown G (2016) Magnetic resonance tumor regression grade and residual mucosal abnormality as predictors for pathological complete response in rectal cancer postneoadjuvant chemoradiotherapy. Dis Colon Rectum 59(10): 925–933. - PubMed
-
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, Wiig JN, Byström P, Bujko K, Glimelius B (2008) Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 26(22): 3687–3694. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical