Long-term Cost Utility of Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome
- PMID: 28934786
- PMCID: PMC8358894
Long-term Cost Utility of Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome
Abstract
Background: Failed back surgery syndrome (FBSS) is a cause of significant morbidity for up to 40% of patients following spine surgery, and is estimated to cost almost $20 billion. Treatment options for these patients currently include conventional medical management (CMM), repeat operation, or spinal cord stimulation (SCS). Much of the published data regarding cost effectiveness of SCS comprise smaller scale randomized controlled trials (RCTs) rather than large databases capturing practices throughout the US. SCS has been shown to have superior outcomes to CMM or repeat spinal operation in several landmark studies, yet there are few large studies examining its long-term economic impact.
Objectives: This study compares health care utilization for SCS compared to other management in patients with FBSS.
Study design: Retrospective.
Setting: Inpatient and outpatient sample.
Methods: Patients with a history of FBSS from 2000 to 2012 were selected. We compared those who received SCS to those who underwent conventional management. A longitudinal analysis was used to model the value of log(cost) in each one year interval using a generalized estimating equations (GEE) model to account for the correlation of the same patient's cost in multiple years. Similarly, a Poisson GEE model with the log link was applied to correlated count outcomes.
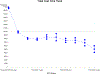
Results: We identified 122,827 FBSS patients. Of these, 5,328 underwent SCS implantation (4.34%) and 117,499 underwent conventional management. Total annual costs decreased over time following implantation of the SCS system, with follow-up analysis at 1, 3, 6, and 9 years. The longitudinal GEE model demonstrated that placement of an SCS system was associated with an initial increase in total costs at the time of implantation (cost ratio [CR]: 1.74; 95% confidence interval [CI]: 1.41, 2.15, P < 0.001), however there was a significant and sustained 68% decrease in cost in the year following SCS placement (CR: 0.32; 95% CI: 0.24, 0.42, P < 0.001) compared to CMM. There was also an aggregate time trend that for each additional year after SCS, cost decreased on average 40% percent annually (CR: 0.60; 95% CI: 0.55, 0.65, P < 0.001), with follow-up up to 1, 3, 6, and 9 years post-procedure.
Limitations: Costs are not correlated with patient outcomes, patients are not stratified in terms of complexity of prior back surgery, as well as inherent limitations of a retrospective analysis.
Conclusions: We found that from 2000 to 2012, only 4.3% of patients across the United States with FBSS were treated with SCS. Long-term total annual costs for these patients were significantly reduced compared to patients with conventional management. Although implantation of an SCS system results in a short-term increase in costs at one year, the subsequent annual cumulative costs were significantly decreased long-term in the following 9 years after implantation. This study combines the largest group of FBSS patients studied to date along with the longest follow-up interval ever analyzed. Since SCS has repeatedly been shown to have superior efficacy to CMM in randomized clinical trials, the current study demonstrating improved long-term health economics at 1, 3, 6, and 9 years supports the long-term cost utility of SCS in the treatment of FBSS patients. Key words: Failed back surgery syndrome, spinal cord stimulation, back pain, leg pain, neuromodulation, FBSS, SCS.
Conflict of interest statement
Disclosure of Interests Statement
Shivanand Lad, MD, PhD has consulted for or received grant support from Medtronic Inc., Boston Scientific and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center that has received research funding from NIH KM1 CA 156687. Siyun Yang, MS was partially supported by UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS). The remaining authors report no conflicts of interest or financial disclosures.
Figures
Similar articles
-
Utilization of spinal cord stimulation in patients with failed back surgery syndrome.Spine (Phila Pa 1976). 2014 May 20;39(12):E719-27. doi: 10.1097/BRS.0000000000000320. Spine (Phila Pa 1976). 2014. PMID: 24718057
-
Cost-Effectiveness and Cost-Utility Analysis of Spinal Cord Stimulation in Patients With Failed Back Surgery Syndrome: Results From the PRECISE Study.Neuromodulation. 2015 Jun;18(4):266-76; discussion 276. doi: 10.1111/ner.12292. Epub 2015 Apr 16. Neuromodulation. 2015. PMID: 25879722 Free PMC article. Clinical Trial.
-
Impact of Insurance Provider on Overall Costs in Failed Back Surgery Syndrome: A Cost Study of 122,827 Patients.Neuromodulation. 2017 Jun;20(4):354-360. doi: 10.1111/ner.12584. Epub 2017 Mar 21. Neuromodulation. 2017. PMID: 28322477 Free PMC article.
-
Counting the costs: case management implications of spinal cord stimulation treatment for failed back surgery syndrome.Prof Case Manag. 2011 Jan-Feb;16(1):27-36. doi: 10.1097/NCM.0b013e3181e9263c. Prof Case Manag. 2011. PMID: 21164332 Review.
-
Spinal Cord Stimulation Meets Them All: An Effective Treatment for Different Pain Conditions. Our Experience and Literature Review.Acta Neurochir Suppl. 2023;135:179-195. doi: 10.1007/978-3-031-36084-8_29. Acta Neurochir Suppl. 2023. PMID: 38153468
Cited by
-
Neuromodulation in chronic pain management: addressing persistent doubts in spinal cord stimulation.J Anesth Analg Crit Care. 2025 Jan 6;5(1):3. doi: 10.1186/s44158-024-00219-6. J Anesth Analg Crit Care. 2025. PMID: 39762994 Free PMC article. Review. No abstract available.
-
A Cost Effectiveness Analysis of Spinal Cord Stimulation versus Conventional Medical Management for the Treatment of Low Back Pain Using Data from DISTINCT RCT and Medical Claims from a U.S. Commercial Payer Database.J Pain Res. 2025 Jun 7;18:2823-2838. doi: 10.2147/JPR.S486759. eCollection 2025. J Pain Res. 2025. PMID: 40502434 Free PMC article.
-
A Systematic Review of the Cost-Utility of Spinal Cord Stimulation for Persistent Low Back Pain in Patients With Failed Back Surgery Syndrome.Global Spine J. 2021 Apr;11(1_suppl):66S-72S. doi: 10.1177/2192568220970163. Global Spine J. 2021. PMID: 33890806 Free PMC article.
-
Interventional Spine and Pain Procedure Credentialing: Guidelines from the American Society of Pain & Neuroscience.J Pain Res. 2021 Sep 8;14:2777-2791. doi: 10.2147/JPR.S309705. eCollection 2021. J Pain Res. 2021. PMID: 34531681 Free PMC article.
-
Healthcare Economics of High Frequency Spinal Cord Stimulation for Painful Diabetic Peripheral Neuropathy.J Diabetes Sci Technol. 2024 May;18(3):635-643. doi: 10.1177/19322968221128321. Epub 2022 Oct 31. J Diabetes Sci Technol. 2024. PMID: 36314587 Free PMC article.
References
-
- Schmidt CO, Raspe H, Pfingsten M, Hasenbring M, Basler HD, Eich W, Kohlmann T. Back pain in the German adult population: prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa 1976) 2007:32: 2005–2011. - PubMed
-
- Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician 2000:61: 1779–1786, 1789–1790. - PubMed
-
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008:8: 8–20. - PubMed
-
- Chan CW, Peng P. Failed back surgery syndrome. Pain Med 2011:12: 577–606. - PubMed
-
- Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, O’Neill C. Failed back surgery: etiology and diagnostic evaluation. Spine J 2003:3: 400–403. - PubMed