Sustained Molecular Pathology Across Episodes and Remission in Major Depressive Disorder
- PMID: 28935211
- PMCID: PMC5705452
- DOI: 10.1016/j.biopsych.2017.08.008
Sustained Molecular Pathology Across Episodes and Remission in Major Depressive Disorder
Abstract
Background: Major depressive disorder (MDD) is a debilitating mental illness and a major cause of lost productivity worldwide. MDD patients often suffer from lifelong recurring episodes of increasing severity, reduced therapeutic response, and shorter remission periods, suggesting the presence of a persistent and potentially progressive pathology.
Methods: Subgenual anterior cingulate cortex postmortem samples from four MDD cohorts (single episode, n = 20; single episode in remission, n = 15; recurrent episode, n = 20; and recurrent episode in remission, n = 15), and one control cohort (n = 20) were analyzed by mass spectrometry-based proteomics (n = 3630 proteins) combined with statistical analyses. The data was investigated for trait and state progressive neuropathologies in MDD using both unbiased approaches and tests of a priori hypotheses.
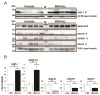
Results: The data provided weak evidence for proteomic differences as a function of state (depressed/remitted) or number of previous episodes. Instead it suggested the presence of persistent MDD effects, regardless of episodes or remitted state, namely on proteomic measures related to presynaptic neurotransmission, synaptic function, cytoskeletal rearrangements, energy metabolism, phospholipid biosynthesis/metabolism, and calcium ion homeostasis. Selected proteins (dihydropyrimidinase-related protein 1, synaptosomal-associated protein 29, glutamate decarboxylase 1, metabotropic glutamate receptor 1, and excitatory amino acid transporter 3) were validated by Western blot analysis. The findings were independent of technical, demographic (sex or age), or other clinical parameters (death by suicide and drug treatment).
Conclusions: Collectively, the results provide evidence for persistent MDD effects across current episodes or remission, in the absence of detectable progressive neuropathology.
Keywords: Bioinformatics; Major depressive disorder; Mass spectrometry; Progressive neuropathologies; Remission; Subgenual cingulate cortex.
Copyright © 2017 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Risks for Major Depression: Searching for Stable Traits.Biol Psychiatry. 2018 Jan 1;83(1):7-8. doi: 10.1016/j.biopsych.2017.10.010. Biol Psychiatry. 2018. PMID: 29173707 Free PMC article. No abstract available.
References
-
- Belmaker RH, Agam G. Major depressive disorder. The New England journal of medicine. 2008;358(1):55–68. - PubMed
-
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60(9):929–37. - PubMed
-
- WHO. World Health Organization - The Global Burden of Disease - 2004 update. WHO Library; 2008.
-
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):593–602. - PubMed
-
- Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Molecular psychiatry. 2013;18(5):595–606. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

