Human Pancreatic Acinar Cells: Proteomic Characterization, Physiologic Responses, and Organellar Disorders in ex Vivo Pancreatitis
- PMID: 28935577
- PMCID: PMC5718097
- DOI: 10.1016/j.ajpath.2017.08.017
Human Pancreatic Acinar Cells: Proteomic Characterization, Physiologic Responses, and Organellar Disorders in ex Vivo Pancreatitis
Abstract
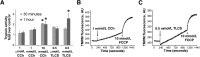
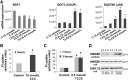
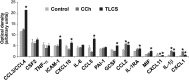
Knowledge of the molecular mechanisms of acute pancreatitis is largely based on studies using rodents. To assess similar mechanisms in humans, we performed ex vivo pancreatitis studies in human acini isolated from cadaveric pancreata from organ donors. Because data on these human acinar preparations are sparse, we assessed their functional integrity and cellular and organellar morphology using light, fluorescence, and electron microscopy; and their proteome by liquid chromatography-tandem mass spectrometry. Acinar cell responses to the muscarinic agonist carbachol (CCh) and the bile acid taurolithocholic acid 3-sulfate were also analyzed. Proteomic analysis of acini from donors of diverse ethnicity showed similar profiles of digestive enzymes and proteins involved in translation, secretion, and endolysosomal function. Human acini preferentially expressed the muscarinic acetylcholine receptor M3 and maintained physiological responses to CCh for at least 20 hours. As in rodent acini, human acini exposed to toxic concentrations of CCh and taurolithocholic acid 3-sulfate responded with trypsinogen activation, decreased cell viability, organelle damage manifest by mitochondrial depolarization, disordered autophagy, and pathological endoplasmic reticulum stress. Human acini also secreted inflammatory mediators elevated in acute pancreatitis patients, including IL-6, tumor necrosis factor-α, IL-1β, chemokine (C-C motif) ligands 2 and 3, macrophage inhibitory factor, and chemokines mediating neutrophil and monocyte infiltration. In conclusion, human cadaveric pancreatic acini maintain physiological functions and have similar pathological responses and organellar disorders with pancreatitis-causing treatments as observed in rodent acini.
Copyright © 2017 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

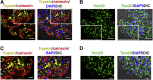
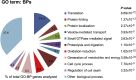
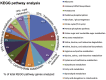


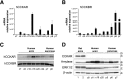

Similar articles
-
Early proteome analysis of rat pancreatic acinar AR42J cells treated with taurolithocholic acid 3-sulfate.Pancreatology. 2012 May-Jun;12(3):248-56. doi: 10.1016/j.pan.2012.02.006. Epub 2012 Feb 16. Pancreatology. 2012. PMID: 22687381
-
Ca2+ Influx Channel Inhibitor SARAF Protects Mice From Acute Pancreatitis.Gastroenterology. 2019 Dec;157(6):1660-1672.e2. doi: 10.1053/j.gastro.2019.08.042. Epub 2019 Sep 4. Gastroenterology. 2019. PMID: 31493399
-
Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology.J Biol Chem. 2017 Apr 7;292(14):5957-5969. doi: 10.1074/jbc.M117.777433. Epub 2017 Feb 27. J Biol Chem. 2017. PMID: 28242761 Free PMC article.
-
Recent Insights Into the Pathogenic Mechanism of Pancreatitis: Role of Acinar Cell Organelle Disorders.Pancreas. 2019 Apr;48(4):459-470. doi: 10.1097/MPA.0000000000001298. Pancreas. 2019. PMID: 30973461 Free PMC article. Review.
-
Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives.Int J Mol Sci. 2020 Jun 3;21(11):4005. doi: 10.3390/ijms21114005. Int J Mol Sci. 2020. PMID: 32503336 Free PMC article. Review.
Cited by
-
Activation of AMP-activated protein kinase attenuates ethanol-induced ER/oxidative stress and lipid phenotype in human pancreatic acinar cells.Biochem Pharmacol. 2020 Oct;180:114174. doi: 10.1016/j.bcp.2020.114174. Epub 2020 Jul 25. Biochem Pharmacol. 2020. PMID: 32717227 Free PMC article.
-
Differential cytotoxicity, ER/oxidative stress, dysregulated AMPKα signaling, and mitochondrial stress by ethanol and its metabolites in human pancreatic acinar cells.Alcohol Clin Exp Res. 2021 May;45(5):961-978. doi: 10.1111/acer.14595. Epub 2021 Apr 2. Alcohol Clin Exp Res. 2021. PMID: 33690904 Free PMC article.
-
The Role of Twist1 in Chronic Pancreatitis-Associated Pancreatic Stellate Cells.Am J Pathol. 2024 Oct;194(10):1879-1897. doi: 10.1016/j.ajpath.2024.06.003. Epub 2024 Jul 18. Am J Pathol. 2024. PMID: 39032603
-
Secondary iron overload induces chronic pancreatitis and ferroptosis of acinar cells in mice.Int J Mol Med. 2023 Jan;51(1):9. doi: 10.3892/ijmm.2022.5212. Epub 2022 Dec 9. Int J Mol Med. 2023. PMID: 36484371 Free PMC article.
-
The Role of Gut Microbiota and Genetic Susceptibility in the Pathogenesis of Pancreatitis.Gut Liver. 2022 Sep 15;16(5):686-696. doi: 10.5009/gnl210362. Epub 2021 Dec 16. Gut Liver. 2022. PMID: 34911043 Free PMC article. Review.
References
-
- Peery A.F., Dellon E.S., Lund J., Crockett S.D., McGowan C.E., Bulsiewicz W.J., Gangarosa L.M., Thiny M.T., Stizenberg K., Morgan D.R., Ringel Y., Kim H.P., Dibonaventura M.D., Carroll C.F., Allen J.K., Cook S.F., Sandler R.S., Kappelman M.D., Shaheen N.J. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143 1179–1187.e1–1187.e3. - PMC - PubMed
-
- Afghani E., Pandol S.J., Shimosegawa T., Sutton R., Wu B.U., Vege S.S., Gorelick F., Hirota M., Windsor J., Lo S.K., Freeman M.L., Lerch M.M., Tsuji Y., Melmed G.Y., Wassef W., Mayerle J. Acute pancreatitis-progress and challenges: a report on an International Symposium. Pancreas. 2015;44:1195–1210. - PMC - PubMed
-
- Pandol S.J., Saluja A.K., Imrie C.W., Banks P.A. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. - PubMed
-
- Lerch M.M., Gorelick F.S. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

