Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension
- PMID: 28935639
- PMCID: PMC5866427
- DOI: 10.1152/ajplung.00296.2017
Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension
Abstract
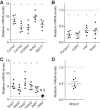
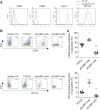
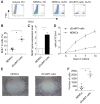
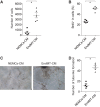
Endothelial-to-mesenchymal transition (EndMT) is a process in which endothelial cells lose polarity and cell-to cell contacts, and undergo a dramatic remodeling of the cytoskeleton. It has been implicated in initiation and progression of pulmonary arterial hypertension (PAH). However, the characteristics of cells which have undergone EndMT cells in vivo have not been reported and so remain unclear. To study this, sugen5416 and hypoxia (SuHx)-induced PAH was established in Cdh5-Cre/Gt(ROSA)26Sortm4(ACTB-tdTomato,EGFP)Luo/J double transgenic mice, in which GFP was stably expressed in pan-endothelial cells. After 3 wk of SuHx, flow cytometry and immunohistochemistry demonstrated CD144-negative and GFP-positive cells (complete EndMT cells) possessed higher proliferative and migratory activity compared with other mesenchymal cells. While CD144-positive and α-smooth muscle actin (α-SMA)-positive cells (partial EndMT cells) continued to express endothelial progenitor cell markers, complete EndMT cells were Sca-1-rich mesenchymal cells with high proliferative and migratory ability. When transferred in fibronectin-coated chamber slides containing smooth muscle media, α-SMA robustly expressed in these cells compared with cEndMT cells that were grown in maintenance media. Demonstrating additional paracrine effects, conditioned medium from isolated complete EndMT cells induced enhanced mesenchymal proliferation and migration and increased angiogenesis compared with conditioned medium from resident mesenchymal cells. Overall, these findings show that EndMT cells could contribute to the pathogenesis of PAH both directly, by transformation into smooth muscle-like cells with higher proliferative and migratory potency, and indirectly, through paracrine effects on vascular intimal and medial proliferation.
Keywords: cell transformation; endothelial-to-mesenchymal transition; paracrine effects; pulmonary arterial hypertension; vascular remodeling.
Figures


References
-
- Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2alpha. Circulation 133: 2447–2458, 2016. doi:10.1161/CIRCULATIONAHA.116.021494. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

