Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension
- PMID: 28935667
- PMCID: PMC5685911
- DOI: 10.1161/CIRCULATIONAHA.117.027589
Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension
Abstract
Background: Immune dysregulation has been linked to occlusive vascular remodeling in pulmonary arterial hypertension (PAH) that is hereditary, idiopathic, or associated with other conditions. Circulating autoantibodies, lung perivascular lymphoid tissue, and elevated cytokines have been related to PAH pathogenesis but without a clear understanding of how these abnormalities are initiated, perpetuated, and connected in the progression of disease. We therefore set out to identify specific target antigens in PAH lung immune complexes as a starting point toward resolving these issues to better inform future application of immunomodulatory therapies.
Methods: Lung immune complexes were isolated and PAH target antigens were identified by liquid chromatography tandem mass spectrometry, confirmed by enzyme-linked immunosorbent assay, and localized by confocal microscopy. One PAH antigen linked to immunity and inflammation was pursued and a link to PAH pathophysiology was investigated by next-generation sequencing, functional studies in cultured monocytes and endothelial cells, and hemodynamic and lung studies in a rat.
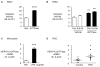
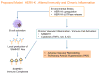
Results: SAM domain and HD domain-containing protein 1 (SAMHD1), an innate immune factor that suppresses HIV replication, was identified and confirmed as highly expressed in immune complexes from 16 hereditary and idiopathic PAH versus 12 control lungs. Elevated SAMHD1 was localized to endothelial cells, perivascular dendritic cells, and macrophages, and SAMHD1 antibodies were prevalent in tertiary lymphoid tissue. An unbiased screen using metagenomic sequencing related SAMHD1 to increased expression of human endogenous retrovirus K (HERV-K) in PAH versus control lungs (n=4). HERV-K envelope and deoxyuridine triphosphate nucleotidohydrolase mRNAs were elevated in PAH versus control lungs (n=10), and proteins were localized to macrophages. HERV-K deoxyuridine triphosphate nucleotidohydrolase induced SAMHD1 and proinflammatory cytokines (eg, interleukin 6, interleukin 1β, and tumor necrosis factor α) in circulating monocytes, pulmonary arterial endothelial cells, and also activated B cells. Vulnerability of pulmonary arterial endothelial cells (PAEC) to apoptosis was increased by HERV-K deoxyuridine triphosphate nucleotidohydrolase in an interleukin 6-independent manner. Furthermore, 3 weekly injections of HERV-K deoxyuridine triphosphate nucleotidohydrolase induced hemodynamic and vascular changes of pulmonary hypertension in rats (n=8) and elevated interleukin 6.
Conclusions: Our study reveals that upregulation of the endogenous retrovirus HERV-K could both initiate and sustain activation of the immune system and cause vascular changes associated with PAH.
Keywords: SAM domain and HD domain-containing protein 1 (SAMHD1); deoxyuridine triphosphate nucleotidohydrolase (dUTPase); human endogenous retrovirus K (HERV-K); pulmonary arterial hypertension (PAH); tertiary lymphoid tissue.
© 2017 American Heart Association, Inc.
Figures





Comment in
-
Human Endogenous Retrovirus K and Pulmonary Arterial Hypertension: A New Take on a Retro Idea.Circulation. 2017 Nov 14;136(20):1936-1938. doi: 10.1161/CIRCULATIONAHA.117.031190. Circulation. 2017. PMID: 29133530 Free PMC article. No abstract available.
Similar articles
-
Monocyte-released HERV-K dUTPase engages TLR4 and MCAM causing endothelial mesenchymal transition.JCI Insight. 2021 Aug 9;6(15):e146416. doi: 10.1172/jci.insight.146416. JCI Insight. 2021. PMID: 34185707 Free PMC article.
-
A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis?J Invest Dermatol. 2011 Dec;131(12):2419-27. doi: 10.1038/jid.2011.217. Epub 2011 Jul 21. J Invest Dermatol. 2011. PMID: 21776007
-
Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension.Am J Respir Crit Care Med. 2012 Nov 1;186(9):897-908. doi: 10.1164/rccm.201202-0335OC. Epub 2012 Sep 6. Am J Respir Crit Care Med. 2012. PMID: 22955318
-
Inflammatory mechanisms in the pathogenesis of pulmonary arterial hypertension.Compr Physiol. 2011 Oct;1(4):1929-41. doi: 10.1002/cphy.c100028. Compr Physiol. 2011. PMID: 23733693 Review.
-
Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation.Int J Mol Sci. 2023 Apr 18;24(8):7472. doi: 10.3390/ijms24087472. Int J Mol Sci. 2023. PMID: 37108634 Free PMC article. Review.
Cited by
-
Identification of immune-associated signatures and potential therapeutic targets for pulmonary arterial hypertension.J Cell Mol Med. 2023 Dec;27(23):3864-3877. doi: 10.1111/jcmm.17962. Epub 2023 Sep 27. J Cell Mol Med. 2023. PMID: 37753829 Free PMC article.
-
Endogenous Retroviruses Unveiled: A Comprehensive Review of Inflammatory Signaling/Senescence-Related Pathways and Therapeutic Strategies.Aging Dis. 2024 May 14;16(2):738-756. doi: 10.14336/AD.2024.0123-1. Aging Dis. 2024. PMID: 38916727 Free PMC article. Review.
-
The crosstalk among autophagy, apoptosis, and pyroptosis in cardiovascular disease.Front Cardiovasc Med. 2022 Oct 28;9:997469. doi: 10.3389/fcvm.2022.997469. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36386383 Free PMC article. Review.
-
Transcriptional Regulation of Endogenous Retroviruses and Their Misregulation in Human Diseases.Int J Mol Sci. 2022 Sep 4;23(17):10112. doi: 10.3390/ijms231710112. Int J Mol Sci. 2022. PMID: 36077510 Free PMC article. Review.
-
Type I interferon activation and endothelial dysfunction in caveolin-1 insufficiency-associated pulmonary arterial hypertension.Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2010206118. doi: 10.1073/pnas.2010206118. Proc Natl Acad Sci U S A. 2021. PMID: 33836561 Free PMC article.
References
-
- Dib H, Tamby MC, Bussone G, Regent A, Berezne A, Lafine C, Broussard C, Simonneau G, Guillevin L, Witko-Sarsat V, Humbert M, Mouthon L. Targets of anti-endothelial cell antibodies in pulmonary hypertension and scleroderma. Eur Respir J. 2012;39:1405–1414. - PubMed
-
- Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. - PubMed
-
- Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI084898/AI/NIAID NIH HHS/United States
- N01 HV028183/HV/NHLBI NIH HHS/United States
- R01 HL138473/HL/NHLBI NIH HHS/United States
- K99 HL135258/HL/NHLBI NIH HHS/United States
- R01 HL082662/HL/NHLBI NIH HHS/United States
- P01 HL108797/HL/NHLBI NIH HHS/United States
- R24 HL123767/HL/NHLBI NIH HHS/United States
- U01 HL107393/HL/NHLBI NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- S10 RR027425/RR/NCRR NIH HHS/United States
- R01 HL122887/HL/NHLBI NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- R01 HL105704/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

