The microanatomic segregation of selection by apoptosis in the germinal center
- PMID: 28935768
- PMCID: PMC5957278
- DOI: 10.1126/science.aao2602
The microanatomic segregation of selection by apoptosis in the germinal center
Abstract
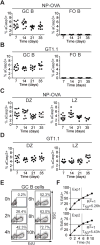
B cells undergo rapid cell division and affinity maturation in anatomically distinct sites in lymphoid organs called germinal centers (GCs). Homeostasis is maintained in part by B cell apoptosis. However, the precise contribution of apoptosis to GC biology and selection is not well defined. We developed apoptosis-indicator mice and used them to visualize, purify, and characterize dying GC B cells. Apoptosis is prevalent in the GC, with up to half of all GC B cells dying every 6 hours. Moreover, programmed cell death is differentially regulated in the light zone and the dark zone: Light-zone B cells die by default if they are not positively selected, whereas dark-zone cells die when their antigen receptors are damaged by activation-induced cytidine deaminase.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Comment in
-
Life, death, and antibodies.Science. 2017 Oct 13;358(6360):171-172. doi: 10.1126/science.aap8728. Science. 2017. PMID: 29026032 No abstract available.
-
Portending death in germinal centers - when B cells know their time is up.Cell Res. 2018 Jan;28(1):5-6. doi: 10.1038/cr.2017.151. Epub 2017 Dec 1. Cell Res. 2018. PMID: 29192678 Free PMC article.
Similar articles
-
AID and caspase 8 shape the germinal center response through apoptosis.J Immunol. 2013 Dec 15;191(12):5840-7. doi: 10.4049/jimmunol.1301776. Epub 2013 Nov 15. J Immunol. 2013. PMID: 24244021
-
Inhibition of the catalytic function of activation-induced cytidine deaminase promotes apoptosis of germinal center B cells in BXD2 mice.Arthritis Rheum. 2011 Jul;63(7):2038-48. doi: 10.1002/art.30257. Arthritis Rheum. 2011. PMID: 21305519 Free PMC article.
-
PTEN-Regulated AID Transcription in Germinal Center B Cells Is Essential for the Class-Switch Recombination and IgG Antibody Responses.Front Immunol. 2018 Feb 28;9:371. doi: 10.3389/fimmu.2018.00371. eCollection 2018. Front Immunol. 2018. PMID: 29541074 Free PMC article.
-
Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination.Crit Rev Immunol. 2007;27(4):367-97. doi: 10.1615/critrevimmunol.v27.i4.60. Crit Rev Immunol. 2007. PMID: 18197815 Free PMC article. Review.
-
Positive Selection in the Light Zone of Germinal Centers.Front Immunol. 2021 Mar 31;12:661678. doi: 10.3389/fimmu.2021.661678. eCollection 2021. Front Immunol. 2021. PMID: 33868314 Free PMC article. Review.
Cited by
-
Guidelines for Regulated Cell Death Assays: A Systematic Summary, A Categorical Comparison, A Prospective.Front Cell Dev Biol. 2021 Mar 4;9:634690. doi: 10.3389/fcell.2021.634690. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748119 Free PMC article. Review.
-
Germinal center expansion but not plasmablast differentiation is proportional to peptide-MHCII density via CD40-CD40L signaling strength.Cell Rep. 2022 May 3;39(5):110763. doi: 10.1016/j.celrep.2022.110763. Cell Rep. 2022. PMID: 35508132 Free PMC article.
-
The Ubiquitin Ligase Itch Skews Light Zone Selection in Germinal Centers.J Immunol. 2023 May 15;210(10):1473-1481. doi: 10.4049/jimmunol.2200824. J Immunol. 2023. PMID: 36929899 Free PMC article.
-
The Role of DNA Repair in Immunological Diversity: From Molecular Mechanisms to Clinical Ramifications.Front Immunol. 2022 Apr 1;13:834889. doi: 10.3389/fimmu.2022.834889. eCollection 2022. Front Immunol. 2022. PMID: 35432317 Free PMC article. Review.
-
Regulation of Decay Accelerating Factor Primes Human Germinal Center B Cells for Phagocytosis.Front Immunol. 2021 Jan 5;11:599647. doi: 10.3389/fimmu.2020.599647. eCollection 2020. Front Immunol. 2021. PMID: 33469456 Free PMC article.
References
-
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. - PubMed
-
- Hauser AE, et al. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

