Sensorimotor computation underlying phototaxis in zebrafish
- PMID: 28935857
- PMCID: PMC5608914
- DOI: 10.1038/s41467-017-00310-3
Sensorimotor computation underlying phototaxis in zebrafish
Abstract
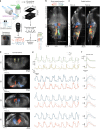
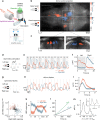
Animals continuously gather sensory cues to move towards favourable environments. Efficient goal-directed navigation requires sensory perception and motor commands to be intertwined in a feedback loop, yet the neural substrate underlying this sensorimotor task in the vertebrate brain remains elusive. Here, we combine virtual-reality behavioural assays, volumetric calcium imaging, optogenetic stimulation and circuit modelling to reveal the neural mechanisms through which a zebrafish performs phototaxis, i.e. actively orients towards a light source. Key to this process is a self-oscillating hindbrain population (HBO) that acts as a pacemaker for ocular saccades and controls the orientation of successive swim-bouts. It further integrates visual stimuli in a state-dependent manner, i.e. its response to visual inputs varies with the motor context, a mechanism that manifests itself in the phase-locked entrainment of the HBO by periodic stimuli. A rate model is developed that reproduces our observations and demonstrates how this sensorimotor processing eventually biases the animal trajectory towards bright regions.Active locomotion requires closed-loop sensorimotor co ordination between perception and action. Here the authors show using behavioural, imaging and modelling approaches that gaze orientation during phototaxis behaviour in larval zebrafish is related to oscillatory dynamics of a neuronal population in the hindbrain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
From behavior to circuit modeling of light-seeking navigation in zebrafish larvae.Elife. 2020 Jan 2;9:e52882. doi: 10.7554/eLife.52882. Elife. 2020. PMID: 31895038 Free PMC article.
-
Efferent modulation of spontaneous lateral line activity during and after zebrafish motor commands.J Neurophysiol. 2019 Dec 1;122(6):2438-2448. doi: 10.1152/jn.00594.2019. Epub 2019 Oct 23. J Neurophysiol. 2019. PMID: 31642405 Free PMC article.
-
Investigation of hindbrain activity during active locomotion reveals inhibitory neurons involved in sensorimotor processing.Sci Rep. 2018 Sep 11;8(1):13615. doi: 10.1038/s41598-018-31968-4. Sci Rep. 2018. PMID: 30206288 Free PMC article.
-
[Visual system and prey capture behavior of larval zebrafish].Yi Chuan. 2013 Apr;35(4):468-76. doi: 10.3724/sp.j.1005.2013.00468. Yi Chuan. 2013. PMID: 23659937 Review. Chinese.
-
From perception to behavior: The neural circuits underlying prey hunting in larval zebrafish.Front Neural Circuits. 2023 Feb 1;17:1087993. doi: 10.3389/fncir.2023.1087993. eCollection 2023. Front Neural Circuits. 2023. PMID: 36817645 Free PMC article. Review.
Cited by
-
A distributed saccade-associated network encodes high velocity conjugate and monocular eye movements in the zebrafish hindbrain.Sci Rep. 2021 Jun 16;11(1):12644. doi: 10.1038/s41598-021-90315-2. Sci Rep. 2021. PMID: 34135354 Free PMC article.
-
Functional Genomics of Epilepsy and Associated Neurodevelopmental Disorders Using Simple Animal Models: From Genes, Molecules to Brain Networks.Front Cell Neurosci. 2019 Dec 13;13:556. doi: 10.3389/fncel.2019.00556. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31920556 Free PMC article. Review.
-
Whole-Brain Neuronal Activity Displays Crackling Noise Dynamics.Neuron. 2018 Dec 19;100(6):1446-1459.e6. doi: 10.1016/j.neuron.2018.10.045. Epub 2018 Nov 16. Neuron. 2018. PMID: 30449656 Free PMC article.
-
Brain-wide Organization of Neuronal Activity and Convergent Sensorimotor Transformations in Larval Zebrafish.Neuron. 2018 Nov 21;100(4):876-890.e5. doi: 10.1016/j.neuron.2018.09.042. Epub 2018 Oct 25. Neuron. 2018. PMID: 30473013 Free PMC article.
-
Predicting modular functions and neural coding of behavior from a synaptic wiring diagram.Nat Neurosci. 2024 Dec;27(12):2443-2454. doi: 10.1038/s41593-024-01784-3. Epub 2024 Nov 22. Nat Neurosci. 2024. PMID: 39578573 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

