Bioenergetic state regulates innate inflammatory responses through the transcriptional co-repressor CtBP
- PMID: 28935892
- PMCID: PMC5608947
- DOI: 10.1038/s41467-017-00707-0
Bioenergetic state regulates innate inflammatory responses through the transcriptional co-repressor CtBP
Abstract
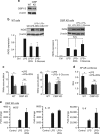
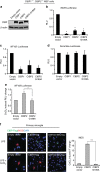
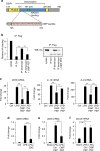
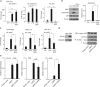
The innate inflammatory response contributes to secondary injury in brain trauma and other disorders. Metabolic factors such as caloric restriction, ketogenic diet, and hyperglycemia influence the inflammatory response, but how this occurs is unclear. Here, we show that glucose metabolism regulates pro-inflammatory NF-κB transcriptional activity through effects on the cytosolic NADH:NAD+ ratio and the NAD(H) sensitive transcriptional co-repressor CtBP. Reduced glucose availability reduces the NADH:NAD+ ratio, NF-κB transcriptional activity, and pro-inflammatory gene expression in macrophages and microglia. These effects are inhibited by forced elevation of NADH, reduced expression of CtBP, or transfection with an NAD(H) insensitive CtBP, and are replicated by a synthetic peptide that inhibits CtBP dimerization. Changes in the NADH:NAD+ ratio regulate CtBP binding to the acetyltransferase p300, and regulate binding of p300 and the transcription factor NF-κB to pro-inflammatory gene promoters. These findings identify a mechanism by which alterations in cellular glucose metabolism can influence cellular inflammatory responses.Several metabolic factors affect cellular glucose metabolism as well as the innate inflammatory response. Here, the authors show that glucose metabolism regulates pro-inflammatory responses through effects on the cytosolic NADH:NAD+ ratio and the NAD(H)-sensitive transcription co-repressor CtBP.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
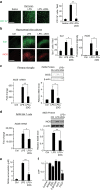
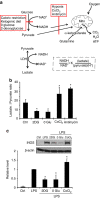
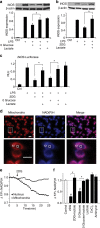
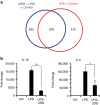
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

