Trap diversity and character evolution in carnivorous bladderworts (Utricularia, Lentibulariaceae)
- PMID: 28935893
- PMCID: PMC5608911
- DOI: 10.1038/s41598-017-12324-4
Trap diversity and character evolution in carnivorous bladderworts (Utricularia, Lentibulariaceae)
Abstract
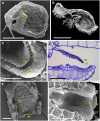
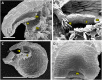
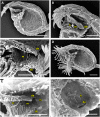
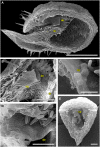
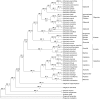
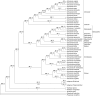
Bladderworts (Utricularia, Lentibulariaceae, Lamiales) constitute the largest genus of carnivorous plants but only aquatic species (about one fifth of the genus) have so far been thoroughly studied as to their suction trap functioning. In this study, we comparatively investigated trap biomechanics in 19 Utricularia species to examine correlations between life-forms, trapping mechanisms, and functional-morphological traits. Our investigations show the existence of two functional trap principles (passive trap in U. multifida vs. active suction traps), and - in active suction traps - three main trapdoor movement types (with several subtypes). The trapdoor movement types and their corresponding functional-morphological features most presumably represent adaptations to the respective habitat. We furthermore give insights into fluid dynamics during suction in three representatives of the main types of trapdoor movement. The results on functional morphology and trapdoor movement were mapped onto a new phylogenetic reconstruction of the genus, derived from the rapidly evolving chloroplast regions trnK, rps16 and trnQ-rps16 and a sampling of 105 Utricularia species in total. We discuss potential scenarios of trap character evolution and species radiation, highlighting possible key innovations that enable such a unique carnivorous lifestyle in different habitats.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

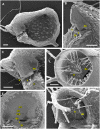


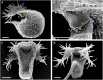
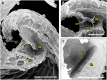
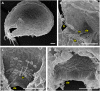





Similar articles
-
A Historical Perspective of Bladderworts (Utricularia): Traps, Carnivory and Body Architecture.Plants (Basel). 2021 Dec 3;10(12):2656. doi: 10.3390/plants10122656. Plants (Basel). 2021. PMID: 34961127 Free PMC article. Review.
-
Fastest predators in the plant kingdom: functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants.AoB Plants. 2015 Nov 24;8:plv140. doi: 10.1093/aobpla/plv140. AoB Plants. 2015. PMID: 26602984 Free PMC article.
-
The Chloroplast Genome of Utricularia reniformis Sheds Light on the Evolution of the ndh Gene Complex of Terrestrial Carnivorous Plants from the Lentibulariaceae Family.PLoS One. 2016 Oct 20;11(10):e0165176. doi: 10.1371/journal.pone.0165176. eCollection 2016. PLoS One. 2016. PMID: 27764252 Free PMC article.
-
Molecular phylogeny of bladderworts: A wide approach of Utricularia (Lentibulariaceae) species relationships based on six plastidial and nuclear DNA sequences.Mol Phylogenet Evol. 2018 Jan;118:244-264. doi: 10.1016/j.ympev.2017.10.010. Epub 2017 Oct 18. Mol Phylogenet Evol. 2018. PMID: 29054811
-
Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification.Ann Bot. 2016 Apr;117(5):811-32. doi: 10.1093/aob/mcv172. Epub 2015 Nov 20. Ann Bot. 2016. PMID: 26589968 Free PMC article. Review.
Cited by
-
Alternative or cytochrome? Respiratory pathways in traps of aquatic carnivorous bladderwort Utricularia reflexa.Plant Signal Behav. 2022 Dec 31;17(1):2134967. doi: 10.1080/15592324.2022.2134967. Plant Signal Behav. 2022. PMID: 36266991 Free PMC article.
-
A Historical Perspective of Bladderworts (Utricularia): Traps, Carnivory and Body Architecture.Plants (Basel). 2021 Dec 3;10(12):2656. doi: 10.3390/plants10122656. Plants (Basel). 2021. PMID: 34961127 Free PMC article. Review.
-
Do Arabinogalactan Proteins Occur in the Transfer Cells of Utricularia dichotoma?Int J Mol Sci. 2024 Jun 16;25(12):6623. doi: 10.3390/ijms25126623. Int J Mol Sci. 2024. PMID: 38928328 Free PMC article.
-
Snapshot prey spectrum analysis of the phylogenetically early-diverging carnivorous Utricularia multifida from U. section Polypompholyx (Lentibulariaceae).PLoS One. 2021 Apr 7;16(4):e0249976. doi: 10.1371/journal.pone.0249976. eCollection 2021. PLoS One. 2021. PMID: 33826676 Free PMC article.
-
Delayed selfing ensures reproductive assurance in Utricularia praeterita and Utricularia babui in Western Ghats.J Plant Res. 2018 Jul;131(4):599-610. doi: 10.1007/s10265-018-1016-y. Epub 2018 Feb 19. J Plant Res. 2018. PMID: 29460199
References
-
- Taylor, P. The genus Utricularia: a taxonomic monograph. Kew Bulletin Additional Series XIV, London, (1989).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

