Zebrafish In-Vivo Screening for Compounds Amplifying Hematopoietic Stem and Progenitor Cells: - Preclinical Validation in Human CD34+ Stem and Progenitor Cells
- PMID: 28935977
- PMCID: PMC5608703
- DOI: 10.1038/s41598-017-12360-0
Zebrafish In-Vivo Screening for Compounds Amplifying Hematopoietic Stem and Progenitor Cells: - Preclinical Validation in Human CD34+ Stem and Progenitor Cells
Abstract
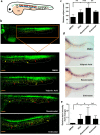
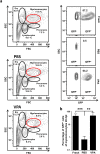
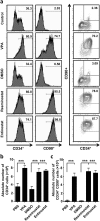
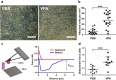
The identification of small molecules that either increase the number and/or enhance the activity of human hematopoietic stem and progenitor cells (hHSPCs) during ex vivo expansion remains challenging. We used an unbiased in vivo chemical screen in a transgenic (c-myb:EGFP) zebrafish embryo model and identified histone deacetylase inhibitors (HDACIs), particularly valproic acid (VPA), as significant enhancers of the number of phenotypic HSPCs, both in vivo and during ex vivo expansion. The long-term functionality of these expanded hHSPCs was verified in a xenotransplantation model with NSG mice. Interestingly, VPA increased CD34+ cell adhesion to primary mesenchymal stromal cells and reduced their in vitro chemokine-mediated migration capacity. In line with this, VPA-treated human CD34+ cells showed reduced homing and early engraftment in a xenograft transplant model, but retained their long-term engraftment potential in vivo, and maintained their differentiation ability both in vitro and in vivo. In summary, our data demonstrate that certain HDACIs lead to a net expansion of hHSPCs with retained long-term engraftment potential and could be further explored as candidate compounds to amplify ex-vivo engineered peripheral blood stem cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
In vivo and in vitro effects of cord blood hematopoietic stem and progenitor cell (HSPC) expansion using valproic acid and/or nicotinamide.Curr Res Transl Med. 2024 Sep;72(3):103444. doi: 10.1016/j.retram.2024.103444. Epub 2024 Mar 1. Curr Res Transl Med. 2024. PMID: 38447268
-
Ex Vivo Expansion of CD34+ CD90+ CD49f+ Hematopoietic Stem and Progenitor Cells from Non-Enriched Umbilical Cord Blood with Azole Compounds.Stem Cells Transl Med. 2018 May;7(5):376-393. doi: 10.1002/sctm.17-0251. Epub 2018 Feb 2. Stem Cells Transl Med. 2018. PMID: 29392885 Free PMC article.
-
Valproic acid affects the engraftment of TPO-expanded cord blood cells in NOD/SCID mice.Exp Cell Res. 2012 Feb 15;318(4):400-7. doi: 10.1016/j.yexcr.2011.11.012. Epub 2011 Dec 6. Exp Cell Res. 2012. PMID: 22166516
-
Advances in umbilical cord blood stem cell expansion and clinical translation.Exp Hematol. 2015 Jul;43(7):498-513. doi: 10.1016/j.exphem.2015.04.011. Epub 2015 May 10. Exp Hematol. 2015. PMID: 25970610 Review.
-
Ex vivo expansion and engraftment potential of cord blood-derived CD34+ cells in NOD/SCID mice.Ann N Y Acad Sci. 2001 Jun;938:9-17. doi: 10.1111/j.1749-6632.2001.tb03569.x. Ann N Y Acad Sci. 2001. PMID: 11458530 Review.
Cited by
-
Epigenetic and Epitranscriptomic Factors Make a Mark on Hematopoietic Stem Cell Development.Curr Stem Cell Rep. 2018 Mar;4(1):22-32. Epub 2018 Feb 3. Curr Stem Cell Rep. 2018. PMID: 29910999 Free PMC article.
-
Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor.Stem Cells Transl Med. 2020 Apr;9(4):531-542. doi: 10.1002/sctm.19-0199. Epub 2020 Jan 17. Stem Cells Transl Med. 2020. PMID: 31950644 Free PMC article.
-
Phenotypic technologies in stem cell biology.Cell Chem Biol. 2021 Mar 18;28(3):257-270. doi: 10.1016/j.chembiol.2021.02.001. Epub 2021 Mar 1. Cell Chem Biol. 2021. PMID: 33651977 Free PMC article. Review.
-
Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis.Ann N Y Acad Sci. 2020 Apr;1466(1):39-50. doi: 10.1111/nyas.14133. Epub 2019 Jun 14. Ann N Y Acad Sci. 2020. PMID: 31199002 Free PMC article. Review.
-
Role of kif2c, A Gene Related to ALL Relapse, in Embryonic Hematopoiesis in Zebrafish.Int J Mol Sci. 2020 Apr 28;21(9):3127. doi: 10.3390/ijms21093127. Int J Mol Sci. 2020. PMID: 32354205 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

