Mouse Anaphylactic Hypotension Is Characterized by Initial Baroreflex Independent Renal Sympathoinhibition Followed by Sustained Renal Sympathoexcitation
- PMID: 28936180
- PMCID: PMC5594092
- DOI: 10.3389/fphys.2017.00669
Mouse Anaphylactic Hypotension Is Characterized by Initial Baroreflex Independent Renal Sympathoinhibition Followed by Sustained Renal Sympathoexcitation
Abstract
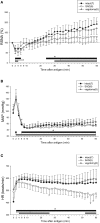
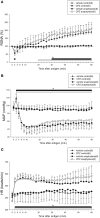
Aim: The hemodynamic response to mouse systemic anaphylaxis is characterized by an initial hypertension followed by sustained hypotension. However, the defense mechanisms of the sympathetic nervous system against this circulatory disturbance is not known. Here, we investigated the renal sympathetic nerve activity (RSNA) response to mouse systemic anaphylaxis, along with the roles of carotid sinus baroreceptor, vagal nerves and the transient receptor potential vanilloid type 1 channel (TRPV1). Methods: Male ovalbumin-sensitized C57BL/6N mice were used under pentobarbital anesthesia. RSNA, systemic arterial pressure (SAP) and heart rate (HR) were continuously measured for 60 min after the antigen injection. Results: Within 3 min after antigen injection, RSNA decreased along with a transient increase in SAP. Thereafter, RSNA showed a progressive increase during sustained hypotension. In contrast, HR continuously increased. Sinoaortic denervation, but not vagotomy, significantly attenuated the renal sympathoexcitation and tachycardia from 30 and 46 min, respectively, after antigen. The responses of RSNA, SAP and HR to anaphylaxis were not affected by pretreatment with a TRPV1 inhibitor, capsazepine, or by genetic knockout of TRPV1. Conclusion: The mouse systemic anaphylaxis causes a biphasic RSNA response with an initial baroreflex-independent decrease and secondary increase. The antigen-induced sympathoexcitation and tachycardia at the late stage are partly mediated by carotid sinus baroreceptors. Either vagal nerve or TRPV1 does not play any significant roles in the RSNA and HR responses in anesthetized mice.
Keywords: TRPV1; anaphylaxis; autonomic nerves; baroreflex; mice; vagal afferents.
Figures


Similar articles
-
Biphasic Renal Sympathetic Response to Hemorrhagic Hypotension in Mice.Shock. 2017 Nov;48(5):576-582. doi: 10.1097/SHK.0000000000000889. Shock. 2017. PMID: 28459715
-
Effects of anesthetics on the renal sympathetic response to anaphylactic hypotension in rats.PLoS One. 2014 Nov 25;9(11):e113945. doi: 10.1371/journal.pone.0113945. eCollection 2014. PLoS One. 2014. PMID: 25423366 Free PMC article.
-
Renal sympathetic and cardiac changes associated with anaphylactic hypotension.Auton Neurosci. 2004 May 31;112(1-2):25-30. doi: 10.1016/j.autneu.2004.03.004. Auton Neurosci. 2004. PMID: 15233927
-
The Role of Lumbar Sympathetic Nerves in Regulation of Blood Flow to Skeletal Muscle during Anaphylactic Hypotension in Anesthetized Rats.PLoS One. 2016 Mar 21;11(3):e0150882. doi: 10.1371/journal.pone.0150882. eCollection 2016. PLoS One. 2016. PMID: 26998924 Free PMC article.
-
Renal sympathetic responses to conflicting baroreceptor inputs: rapid ventricular pacing in dogs.J Physiol. 1993 Nov;471:365-78. doi: 10.1113/jphysiol.1993.sp019905. J Physiol. 1993. PMID: 8120811 Free PMC article.
Cited by
-
GFAP-Positive Progenitor Cell Production is Concentrated in Specific Encephalic Regions in Young Adult Mice.Neurosci Bull. 2018 Oct;34(5):769-778. doi: 10.1007/s12264-018-0228-4. Epub 2018 Apr 16. Neurosci Bull. 2018. PMID: 29663175 Free PMC article.
-
The antidepressive mechanism of Longya Lilium combined with Fluoxetine in mice with depression-like behaviors.NPJ Syst Biol Appl. 2024 Jan 13;10(1):5. doi: 10.1038/s41540-024-00329-5. NPJ Syst Biol Appl. 2024. PMID: 38218856 Free PMC article.
-
4-Phenylbutyrate Mitigates the Motor Impairment and Dopaminergic Neuronal Death During Parkinson's Disease Pathology via Targeting VDAC1 Mediated Mitochondrial Function and Astrocytes Activation.Neurochem Res. 2022 Nov;47(11):3385-3401. doi: 10.1007/s11064-022-03691-0. Epub 2022 Aug 3. Neurochem Res. 2022. PMID: 35922743
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

