Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1
- PMID: 28936211
- PMCID: PMC5594076
- DOI: 10.3389/fimmu.2017.01097
Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1
Abstract
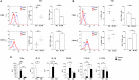
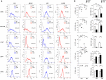
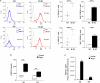
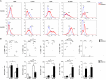
Macrophages (Mf) are a heterogeneous population of tissue-resident professional phagocytes and a major component of the leukocyte infiltrate at sites of inflammation, infection, and tumor growth. They can undergo diverse forms of activation in response to environmental factors, polarizing into specialized functional subsets. A common hallmark of the pathologic environment is represented by hypoxia. The impact of hypoxia on human Mf polarization has not been fully established. The objective of this study was to elucidate the effects of a hypoxic environment reflecting that occurring in vivo in diseased tissues on the ability of human Mf to polarize into classically activated (proinflammatory M1) and alternatively activated (anti-inflammatory M2) subsets. We present data showing that hypoxia hinders Mf polarization toward the M1 phenotype by decreasing the expression of T cell costimulatory molecules and chemokine homing receptors and the production of proinflammatory, Th1-priming cytokines typical of classical activation, while promoting their acquisition of phenotypic and secretory features of alternative activation. Furthermore, we identify the triggering receptor expressed on myeloid cells (TREM)-1, a member of the Ig-like immunoregulatory receptor family, as a hypoxia-inducible gene in Mf and demonstrate that its engagement by an agonist Ab reverses the M2-polarizing effect of hypoxia imparting a M1-skewed phenotype to Mf. Finally, we provide evidence that Mf infiltrating the inflamed hypoxic joints of children affected by oligoarticular juvenile idiopatic arthritis express high surface levels of TREM-1 associated with predominant M1 polarization and suggest the potential of this molecule in driving M1 proinflammatory reprogramming in the hypoxic synovial environment.
Keywords: hypoxia; immunoregulatory receptors; inflammation; macrophages; polarization.
Figures




Similar articles
-
Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells.Oncol Lett. 2019 Dec;18(6):5871-5878. doi: 10.3892/ol.2019.10956. Epub 2019 Oct 3. Oncol Lett. 2019. PMID: 31788060 Free PMC article.
-
Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
-
Regulation of Langerhans cell functions in a hypoxic environment.J Mol Med (Berl). 2016 Aug;94(8):943-55. doi: 10.1007/s00109-016-1400-9. Epub 2016 Mar 10. J Mol Med (Berl). 2016. PMID: 26960761
-
Chronic hypoxia reprograms human immature dendritic cells by inducing a proinflammatory phenotype and TREM-1 expression.Eur J Immunol. 2013 Apr;43(4):949-66. doi: 10.1002/eji.201242709. Epub 2013 Mar 6. Eur J Immunol. 2013. PMID: 23436478
-
Diversity and plasticity of microglial cells in psychiatric and neurological disorders.Pharmacol Ther. 2015 Oct;154:21-35. doi: 10.1016/j.pharmthera.2015.06.010. Epub 2015 Jun 27. Pharmacol Ther. 2015. PMID: 26129625 Review.
Cited by
-
The interplay between the airway epithelium and tissue macrophages during the SARS-CoV-2 infection.Front Immunol. 2022 Oct 6;13:991991. doi: 10.3389/fimmu.2022.991991. eCollection 2022. Front Immunol. 2022. PMID: 36275746 Free PMC article.
-
Combination treatment of mannose and GalNAc conjugated small interfering RNA protects against lethal Marburg virus infection.Mol Ther. 2023 Jan 4;31(1):269-281. doi: 10.1016/j.ymthe.2022.09.009. Epub 2022 Sep 15. Mol Ther. 2023. PMID: 36114672 Free PMC article.
-
Differential polarization and the expression of efferocytosis receptor MerTK on M1 and M2 macrophages isolated from coronary artery disease patients.BMC Immunol. 2021 Mar 24;22(1):21. doi: 10.1186/s12865-021-00410-2. BMC Immunol. 2021. PMID: 33761885 Free PMC article.
-
Equine peripheral blood CD14+ monocyte-derived macrophage in-vitro characteristics after GM-CSF pretreatment and LPS+IFN-γ or IL-4+IL-10 differentiation.Vet Immunol Immunopathol. 2023 Jan;255:110534. doi: 10.1016/j.vetimm.2022.110534. Epub 2022 Dec 7. Vet Immunol Immunopathol. 2023. PMID: 36502640 Free PMC article.
-
Acute AT1R blockade prevents isoproterenol-induced injury in mdx hearts.J Mol Cell Cardiol. 2019 Mar;128:51-61. doi: 10.1016/j.yjmcc.2019.01.013. Epub 2019 Jan 19. J Mol Cell Cardiol. 2019. PMID: 30664850 Free PMC article.
References
-
- Bosco MC, Varesio L. Monocytic cell gene regulation by the hypoxic synovial environment in juvenile idiopathic arthritis: implications for disease pathogenesis. J Clin Rheumatol Musculoskelet Med (2010) 1:47–55.
LinkOut - more resources
Full Text Sources
Other Literature Sources

