The effects of CO2 and H2 on CO metabolism by pure and mixed microbial cultures
- PMID: 28936234
- PMCID: PMC5603099
- DOI: 10.1186/s13068-017-0910-1
The effects of CO2 and H2 on CO metabolism by pure and mixed microbial cultures
Abstract
Background: Syngas fermentation, the bioconversion of CO, CO2, and H2 to biofuels and chemicals, has undergone considerable optimization for industrial applications. Even more, full-scale plants for ethanol production from syngas fermentation by pure cultures are being built worldwide. The composition of syngas depends on the feedstock gasified and the gasification conditions. However, it remains unclear how different syngas mixtures affect the metabolism of carboxidotrophs, including the ethanol/acetate ratios. In addition, the potential application of mixed cultures in syngas fermentation and their advantages over pure cultures have not been deeply explored. In this work, the effects of CO2 and H2 on the CO metabolism by pure and mixed cultures were studied and compared. For this, a CO-enriched mixed culture and two isolated carboxidotrophs were grown with different combinations of syngas components (CO, CO:H2, CO:CO2, or CO:CO2:H2).
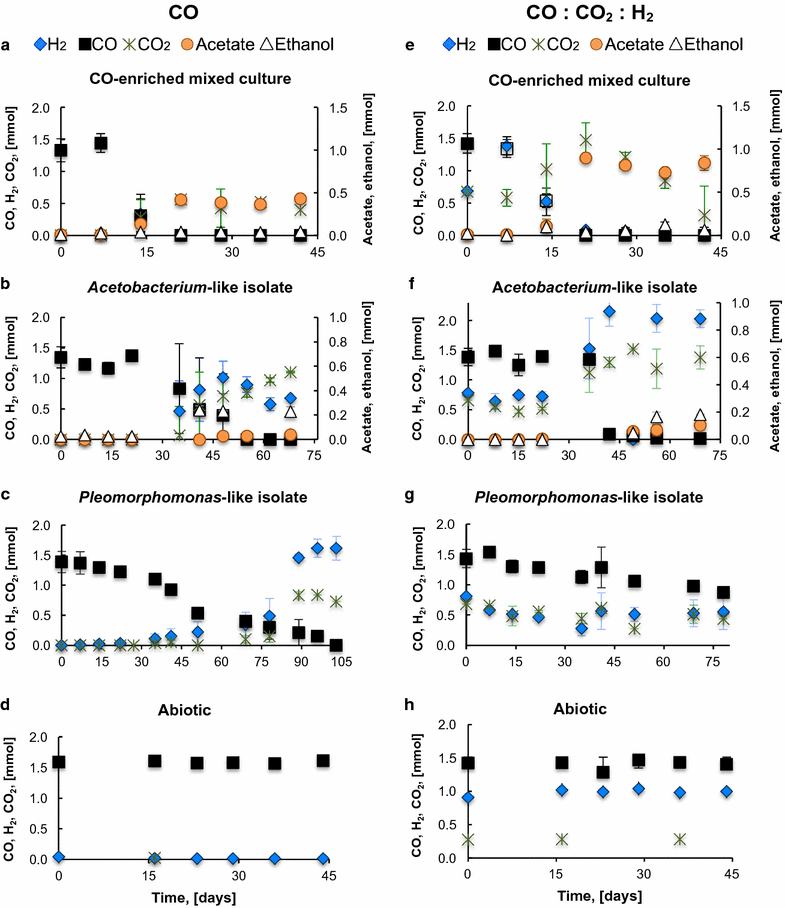
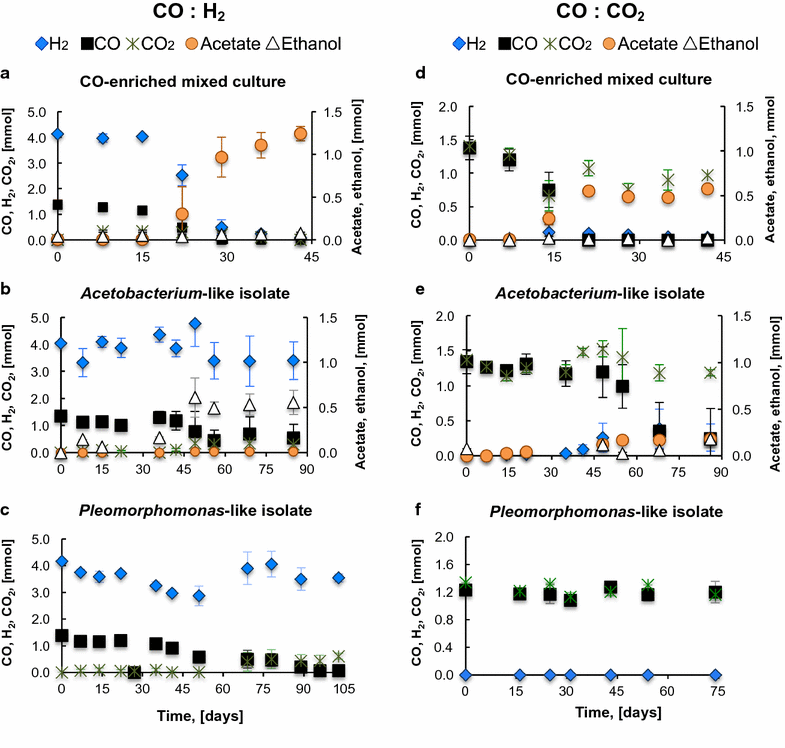
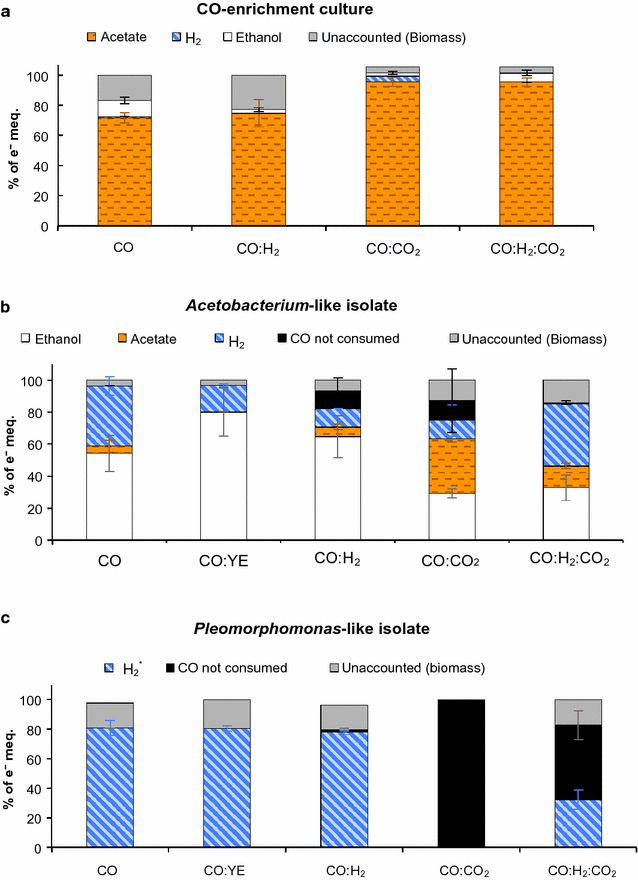
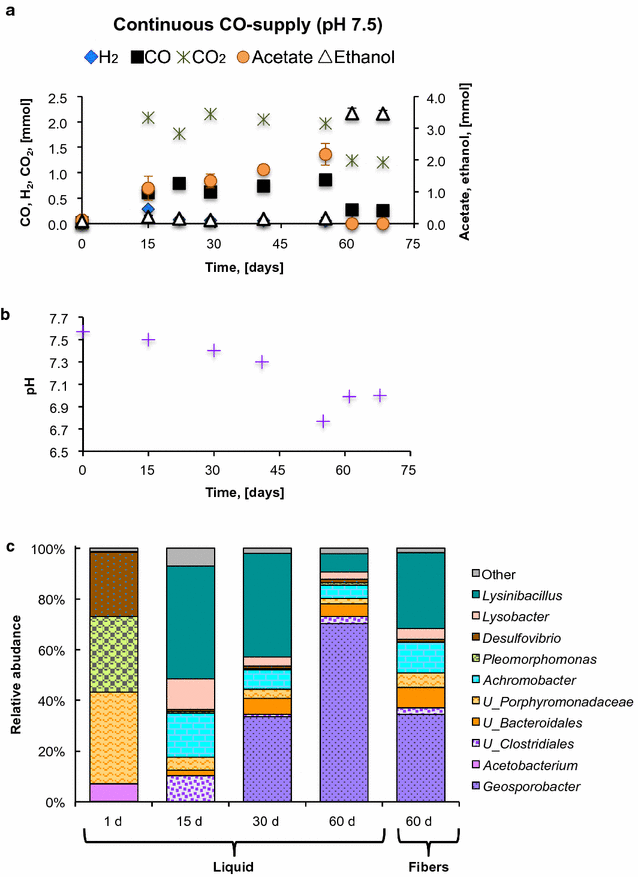
Results: The CO metabolism of the mixed culture was somehow affected by the addition of CO2 and/or H2, but the pure cultures were more sensitive to changes in gas composition than the mixed culture. CO2 inhibited CO oxidation by the Pleomorphomonas-like isolate and decreased the ethanol/acetate ratio by the Acetobacterium-like isolate. H2 did not inhibit ethanol or H2 production by the Acetobacterium and Pleomorphomonas isolates, respectively, but decreased their CO consumption rates. As part of the mixed culture, these isolates, together with other microorganisms, consumed H2 and CO2 (along with CO) for all conditions tested and at similar CO consumption rates (2.6 ± 0.6 mmol CO L-1 day-1), while maintaining overall function (acetate production). Providing a continuous supply of CO by membrane diffusion caused the mixed culture to switch from acetate to ethanol production, presumably due to the increased supply of electron donor. In parallel with this change in metabolic function, the structure of the microbial community became dominated by Geosporobacter phylotypes, instead of Acetobacterium and Pleomorphomonas phylotypes.
Conclusions: These results provide evidence for the potential of mixed-culture syngas fermentation, since the CO-enriched mixed culture showed high functional redundancy, was resilient to changes in syngas composition, and was capable of producing acetate or ethanol as main products of CO metabolism.
Keywords: Acetobacterium; Bioethanol; CO-enriched mixed culture; Carbon monoxide; Geosporobacter; Pleomorphomonas; Syngas.
Figures
References
-
- Vega JL, Prieto S, Elmore BB, Clausen EC, Gladdy JL. The biological production of ethanol from synthesis gas. Appl Biochem Biotechnol. 1989;20–21:781–797. doi: 10.1007/BF02936525. - DOI
-
- Grethlein AJ, Worden RM, Jain MK, Datta R. Evidence for production of n-butanol from carbon monoxide by Butyribacterium methylotrophicum. J Ferment Bioeng. 1991;72:58–60. doi: 10.1016/0922-338X(91)90147-9. - DOI
-
- Luo G, Wang W, Angelidaki I. Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: process performance and microbial ecology. Environ Sci Technol. 2013;47:10685–10693. - PubMed
-
- Shen Y, Brown R, Wen Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl Energy. 2014;136:68–76. doi: 10.1016/j.apenergy.2014.08.117. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

