Modulating the Catalytic Activity of Cerium Oxide Nanoparticles with the Anion of the Precursor Salt
- PMID: 28936278
- PMCID: PMC5602578
- DOI: 10.1021/acs.jpcc.7b05725
Modulating the Catalytic Activity of Cerium Oxide Nanoparticles with the Anion of the Precursor Salt
Abstract
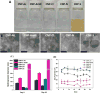
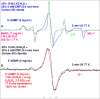
In this work, we tested our hypothesis that surface chemistry and antioxidant properties of cerium nanoparticles (CNPs) are affected by presence of counterions. We first employed various precursor cerium (III) (Ce(III)) salts with different counterions (acetate, nitrate, chloride, sulfate) to synthesize CNPs following the same wet chemical methodology. Electron spin resonance (ESR) studies provided evidence for the formation of radicals from counterions (e.g., NO3•2- from reduction of NO3- in CNPs synthesized from Ce(III) nitrate). Physicochemical properties of these CNPs, e.g., dispersion stability, hydrodynamic size, signature surface chemistry, SOD-mimetic activity, and oxidation potentials were found to be significantly affected by the anions of the precursor salts. CNPs synthesized from Ce(III) nitrate and Ce(III) chloride exhibited higher extent of SOD-mimetic activities. Therefore, these CNPs were studied extensively employing in-situ UV-Visible spectroelectrochemistry and changing the counterion concentrations affected the oxidation potentials of these CNPs. Thus, the physicochemical and antioxidant properties of CNPs can be modulated by anions of the precursor. Furthermore, our ESR studies present evidence of the formation of guanine cation radical (G•+) in 5'-dGMP via UV-photoionization at 77 K in the presence of CNPs synthesized from Ce(III) nitrate and chloride and CNPs act as the scavenger of radiation-produced electrons.
Conflict of interest statement
Notes The authors declare no competing financial interests.
Figures







References
-
- Lohse SE, Murphy CJ. Applications of Colloidal Inorganic Nanoparticles: From Medicine to Energy. J Am Chem Soc. 2012;134:15607–15620. - PubMed
-
- Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium Oxide Nanoparticles: Applications and Prospects in Nanomedicine. Nanomedicine. 2013;8:1483–1508. - PubMed
-
- Yang JC, Kim HJ, Kim T. Study of Polishing Characteristics of Monodisperse Ceria Abrasive in Chemical Mechanical Planarization. J Electrochem Soc. 2010;157:H235–H240.
-
- Rodriguez JA, Graciani J, Evans J, Park JB, Yang F, Stacchiola D, Senanayake SD, Ma S, Pérez M, Liu P. Water‐Gas Shift Reaction on a Highly Active Inverse CeOx/Cu (111) Catalyst: Unique Role of Ceria Nanoparticles. Angew Chem. 2009;121:8191–8194. - PubMed
-
- Masui T, Yamamoto M, Sakata T, Mori H, Adachi G-Y. Synthesis of BN-coated CeO2 Fine Powder as a New UV Blocking Material. J Materials Chem. 2000;10:353–357.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
