pH-dependent binding of guests in the cavity of a polyhedral coordination cage: reversible uptake and release of drug molecules
- PMID: 28936311
- PMCID: PMC5588781
- DOI: 10.1039/c4sc02090a
pH-dependent binding of guests in the cavity of a polyhedral coordination cage: reversible uptake and release of drug molecules
Abstract
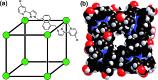
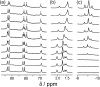
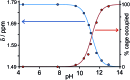
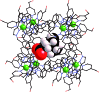
A range of organic molecules with acidic or basic groups exhibit strong pH-dependent binding inside the cavity of a polyhedral coordination cage. Guest binding in aqueous solution is dominated by a hydrophobic contribution which is compensated by stronger solvation when the guests become cationic (by protonation) or anionic (by deprotonation). The Parkinson's drug 1-amino-adamantane ('amantadine') binds with an association constant of 104 M-1 in the neutral form (pH greater than 11), but the stability of the complex is reduced by three orders of magnitude when the guest is protonated at lower pH. Monitoring the uptake of the guests into the cage cavity was facilitated by the large upfield shift for the 1H NMR signals of bound guests due to the paramagnetism of the host. Although the association constants are generally lower, guests of biological significance such as aspirin and nicotine show similar behaviour, with a substantial difference between neutral (strongly binding) and charged (weakly binding) forms, irrespective of the sign of the charged species. pH-dependent binding was observed for a range of guests with different functional groups (primary and tertiary amines, pyridine, imidazole and carboxylic acids), so that the pH-swing can be tuned anywhere in the range of 3.5-11. The structure of the adamantane-1-carboxylic acid complex was determined by X-ray crystallography: the oxygen atoms of the guest form CH···O hydrogen bonds with one of two equivalent pockets on the internal surface of the host. Reversible uptake and release of guests as a function of pH offers interesting possibilities in any application where controlled release of a molecule following an external stimulus is required.
Figures




Similar articles
-
pH-Controlled selection between one of three guests from a mixture using a coordination cage host.Chem Sci. 2015 Jul 15;6(7):4025-4028. doi: 10.1039/c5sc01475a. Epub 2015 May 7. Chem Sci. 2015. PMID: 28717464 Free PMC article.
-
Coordination-cage binding and catalysed hydrolysis of organophosphorus chemical warfare agent simulants.RSC Adv. 2024 Aug 19;14(36):26032-26042. doi: 10.1039/d4ra04705b. eCollection 2024 Aug 16. RSC Adv. 2024. PMID: 39161455 Free PMC article.
-
Coordination Cages Based on Bis(pyrazolylpyridine) Ligands: Structures, Dynamic Behavior, Guest Binding, and Catalysis.Acc Chem Res. 2018 Sep 18;51(9):2073-2082. doi: 10.1021/acs.accounts.8b00261. Epub 2018 Aug 7. Acc Chem Res. 2018. PMID: 30085644
-
Coupling of electron transfer to proton uptake at the Q(B) site of the bacterial reaction center: a perspective from FTIR difference spectroscopy.Biochim Biophys Acta. 2008 Oct;1777(10):1229-48. doi: 10.1016/j.bbabio.2008.06.012. Epub 2008 Jul 11. Biochim Biophys Acta. 2008. PMID: 18671937 Review.
-
Cyclodextrin binding constants as a function of pH for compounds with multiple pKa values.Int J Pharm. 2020 Jul 30;585:119493. doi: 10.1016/j.ijpharm.2020.119493. Epub 2020 Jun 2. Int J Pharm. 2020. PMID: 32502687 Review.
Cited by
-
Application of Crystalline Matrices for the Structural Determination of Organic Molecules.ACS Cent Sci. 2021 Mar 24;7(3):406-414. doi: 10.1021/acscentsci.0c01492. Epub 2021 Feb 17. ACS Cent Sci. 2021. PMID: 33791424 Free PMC article. Review.
-
A fluorescent calixarene-based dimeric capsule constructed via a MII-terpyridine interaction: cage structure, inclusion properties and drug release.RSC Adv. 2018 Jun 20;8(40):22530-22535. doi: 10.1039/c8ra02146e. eCollection 2018 Jun 19. RSC Adv. 2018. PMID: 35539710 Free PMC article.
-
Capture of Singlet Oxygen Modulates Host-Guest Behavior of Coordination Cages.Angew Chem Int Ed Engl. 2023 Sep 25;62(39):e202309589. doi: 10.1002/anie.202309589. Epub 2023 Aug 23. Angew Chem Int Ed Engl. 2023. PMID: 37610599 Free PMC article.
-
Temperature Controls Guest Uptake and Release from Zn4L4 Tetrahedra.J Am Chem Soc. 2019 Sep 18;141(37):14534-14538. doi: 10.1021/jacs.9b07307. Epub 2019 Sep 9. J Am Chem Soc. 2019. PMID: 31478658 Free PMC article.
-
Biomedically Relevant Self-Assembled Metallacycles and Metallacages.J Am Chem Soc. 2019 Sep 11;141(36):14005-14020. doi: 10.1021/jacs.9b06222. Epub 2019 Aug 29. J Am Chem Soc. 2019. PMID: 31419112 Free PMC article. Review.
References
-
- Rebek J. Acc. Chem. Res. 2009;42:1660. - PubMed
- Cram D. J. Angew. Chem., Int. Ed. Engl. 1988;27:1009.
- Rieth S., Hermann K., Wang B.-Y., Badjić J. Chem. Soc. Rev. 2011;40:1609. - PubMed
- Hof F., Craig S. L., Nucjolls C., Rebek J. J. Angew. Chem., Int. Ed. 2002;41:1488. - PubMed
- Hooley R. J., Rebek J. Chem. Biol. 2009;16:255. - PMC - PubMed
- Cram D. J. Nature. 1992;356:29.
- Yoshizawa M., Klosterman J. Chem. Soc. Rev. 2014;43:1885. - PubMed
- Collet A., Dutasta J.-P., Lozach B., Canceill J. Top. Curr. Chem. 1993;165:103.
- Conn M. M., Rebek J. Chem. Rev. 1997;97:1647. - PubMed
- Sherman J. C. Tetrahedron. 1995;51:3395.
- Ajami D., Rebek J. Acc. Chem. Res. 2013;46:990. - PubMed
- Adriaenssens L., Ballester P. Chem. Soc. Rev. 2013;42:3261. - PubMed
-
- Fiedler D., Leung D. H., Bergman R. G., Raymond K. N. Acc. Chem. Res. 2005;38:349. - PubMed
- Fujita M., Tominaga M., Hori A., Therrien B. Acc. Chem. Res. 2005;38:369. - PubMed
- Seidel S. R., Stang P. J. Acc. Chem. Res. 2002;35:972. - PubMed
- Hamilton T. D., MacGillivray L. R. Cryst. Growth Des. 2004;4:419.
- Ward M. D. Chem. Commun. 2009:4487. - PubMed
- Perry J. J., Perman J. A., Zaworotko M. J. Chem. Soc. Rev. 2009;38:1400. - PubMed
- Alvarez A. Dalton Trans. 2006:2209. - PubMed
- Amouri H., Desmarets C., Moussa J. Chem. Rev. 2012;112:2015. - PubMed
- Williams A. F. Coord. Chem. Rev. 2011;255:2104.
- Laughrey Z., Gibb B. Chem. Soc. Rev. 2011;40:363. - PubMed
- Jin P., Dalgarno S. J., Atwood J. L. Coord. Chem. Rev. 2012;254:1760.
- Inokuma Y., Kawano M., Fujita M. Nat. Chem. 2011;3:349. - PubMed
- Pluth M. D., Bergman R. G., Raymond K. N. Acc. Chem. Res. 2009;42:1650. - PubMed
- Breiner B., Clegg J. K., Nitschke J. R. Chem. Sci. 2011;2:51.
- Smulders M. M. J., Riddell I. A., Browne C., Nitschke J. R. Chem. Soc. Rev. 2013;42:1728. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

