DNA based multi-copper ions assembly using combined pyrazole and salen ligandosides
- PMID: 28936312
- PMCID: PMC5588782
- DOI: 10.1039/c4sc01567c
DNA based multi-copper ions assembly using combined pyrazole and salen ligandosides
Abstract
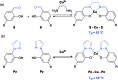
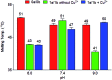
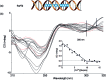
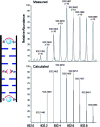
The DNA structure is an ideal building block for the construction of functional nano-objects. In this direction, metal coordinating base pairs (ligandosides) are an appealing tool for the future specific functionalization of such nano-objects. We present here a study, in which we combine the metal ion coordinating pyrazole ligandoside with the interstrand crosslinking salen ligandoside system. We show that both ligandosides, when combined, are able to create stable multi-copper ion complexing DNA double helix structures in a cooperative fashion.
Figures




Similar articles
-
Synthesis and properties of a Cu(II) complexing pyrazole ligandoside in DNA.Chem Commun (Camb). 2014 Jan 14;50(4):409-11. doi: 10.1039/c3cc47561a. Chem Commun (Camb). 2014. PMID: 24263097
-
Metal-salen-base-pair complexes inside DNA: complexation overrides sequence information.Chemistry. 2006 Nov 24;12(34):8708-18. doi: 10.1002/chem.200600558. Chemistry. 2006. PMID: 16983709
-
Synthesis and properties of the simplified nucleic acid glycol nucleic acid.Acc Chem Res. 2010 Aug 17;43(8):1092-102. doi: 10.1021/ar900292q. Acc Chem Res. 2010. PMID: 20405911
-
Hybridization-specific chemical reactions to create interstrand crosslinking and threaded structures of nucleic acids.Org Biomol Chem. 2022 Jun 15;20(23):4699-4708. doi: 10.1039/d2ob00551d. Org Biomol Chem. 2022. PMID: 35622064 Review.
-
Al(Salen) Metal Complexes in Stereoselective Catalysis.Molecules. 2019 May 2;24(9):1716. doi: 10.3390/molecules24091716. Molecules. 2019. PMID: 31052604 Free PMC article. Review.
Cited by
-
Chiral-at-Metal Silver-Mediated Base Pairs: Metal-Centred Chirality versus DNA Helical Chirality.Chemistry. 2023 Jan 12;29(3):e202202630. doi: 10.1002/chem.202202630. Epub 2022 Nov 22. Chemistry. 2023. PMID: 36219466 Free PMC article.
-
Addressing the properties of "Metallo-DNA" with a Ag(i)-mediated supramolecular duplex.Chem Sci. 2019 Feb 8;10(11):3186-3195. doi: 10.1039/c8sc05103h. eCollection 2019 Mar 21. Chem Sci. 2019. PMID: 30996900 Free PMC article.
-
Controllable DNA strand displacement by independent metal-ligand complexation.Chem Sci. 2021 May 18;12(25):8698-8705. doi: 10.1039/d1sc01041g. eCollection 2021 Jul 1. Chem Sci. 2021. PMID: 34257868 Free PMC article.
-
New Benchmark in DNA-Based Asymmetric Catalysis: Prevalence of Modified DNA/RNA Hybrid Systems.JACS Au. 2022 Aug 2;2(8):1910-1917. doi: 10.1021/jacsau.2c00271. eCollection 2022 Aug 22. JACS Au. 2022. PMID: 36032523 Free PMC article.
-
Stable Hg(II)-mediated base pairs with a phenanthroline-derived nucleobase surrogate in antiparallel-stranded DNA.J Biol Inorg Chem. 2020 Jun;25(4):647-654. doi: 10.1007/s00775-020-01788-x. Epub 2020 Apr 11. J Biol Inorg Chem. 2020. PMID: 32277288 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

