Small endohedral metallofullerenes: exploration of the structure and growth mechanism in the Ti@C2 n (2 n = 26-50) family
- PMID: 28936315
- PMCID: PMC5590485
- DOI: 10.1039/c4sc02268h
Small endohedral metallofullerenes: exploration of the structure and growth mechanism in the Ti@C2 n (2 n = 26-50) family
Abstract
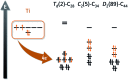
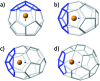
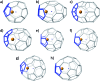
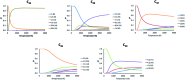
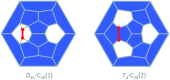
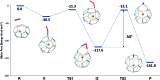
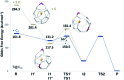
The formation of the smallest fullerene, C28, was recently reported using gas phase experiments combined with high-resolution FT-ICR mass spectrometry. An internally located group IV metal stabilizes the highly strained non-IPR C28 cage by charge transfer (IPR = isolated pentagon rule). Ti@C44 also appeared as a prominent peak in the mass spectra, and U@C28 was demonstrated to form by a bottom-up growth mechanism. We report here a computational analysis using standard DFT calculations and Car-Parrinello MD simulations for the family of the titled compounds, aiming to identify the optimal cage for each endohedral fullerene and to unravel key aspects of the intriguing growth mechanisms of fullerenes. We show that all the optimal isomers from C26 to C50 are linked by a simple C2 insertion, with the exception of a few carbon cages that require an additional C2 rearrangement. The ingestion of a C2 unit is always an exergonic/exothermic process that can occur through a rather simple mechanism, with the most energetically demanding step corresponding to the closure of the carbon cage. The large formation abundance observed in mass spectra for Ti@C28 and Ti@C44 can be explained by the special electronic properties of these cages and their higher relative stabilities with respect to C2 reactivity. We further verify that extrusion of C atoms from an already closed fullerene is much more energetically demanding than forming the fullerene by a bottom-up mechanism. Independent of the formation mechanism, the present investigations strongly support that, among all the possible isomers, the most stable, smaller non-IPR carbon cages are formed, a conclusion that is also valid for medium and large cages.
Figures


Similar articles
-
The smallest stable fullerene, M@C28 (m = Ti, Zr, U): stabilization and growth from carbon vapor.J Am Chem Soc. 2012 Jun 6;134(22):9380-9. doi: 10.1021/ja302398h. Epub 2012 May 24. J Am Chem Soc. 2012. PMID: 22519801
-
Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity.Acc Chem Res. 2019 Jul 16;52(7):1824-1833. doi: 10.1021/acs.accounts.9b00229. Epub 2019 Jul 1. Acc Chem Res. 2019. PMID: 31260256
-
Synthesis and Characterization of Non-Isolated-Pentagon-Rule Actinide Endohedral Metallofullerenes U@ C1(17418)-C76, U@ C1(28324)-C80, and Th@ C1(28324)-C80: Low-Symmetry Cage Selection Directed by a Tetravalent Ion.J Am Chem Soc. 2018 Dec 26;140(51):18039-18050. doi: 10.1021/jacs.8b10435. Epub 2018 Dec 4. J Am Chem Soc. 2018. PMID: 30453733
-
The stabilization of fused-pentagon fullerene molecules.Nat Chem. 2009 Sep;1(6):450-60. doi: 10.1038/nchem.329. Epub 2009 Aug 24. Nat Chem. 2009. PMID: 21378913 Review.
-
Strain Release of Fused Pentagons in Fullerene Cages by Chemical Functionalization.Angew Chem Int Ed Engl. 2020 Jan 13;59(3):1048-1073. doi: 10.1002/anie.201901678. Epub 2019 Nov 8. Angew Chem Int Ed Engl. 2020. PMID: 30884036 Review.
Cited by
-
Crystallographic identification of Eu@C2n (2n = 88, 86 and 84): completing a transformation map for existing metallofullerenes.Chem Sci. 2018 Dec 17;10(7):2153-2158. doi: 10.1039/c8sc04906h. eCollection 2019 Feb 21. Chem Sci. 2018. PMID: 30881639 Free PMC article.
-
Endohedral Metallofullerenes: Unveiling Synthesis Mechanisms and Advancing Photoelectric Energy Conversion Applications.Top Curr Chem (Cham). 2025 Mar 14;383(2):14. doi: 10.1007/s41061-025-00500-4. Top Curr Chem (Cham). 2025. PMID: 40085336 Review.
-
Are U-U Bonds Inside Fullerenes Really Unwilling Bonds?J Am Chem Soc. 2023 Mar 29;145(12):6710-6718. doi: 10.1021/jacs.2c12346. Epub 2023 Mar 5. J Am Chem Soc. 2023. PMID: 36872864 Free PMC article.
-
Self-assembly of endohedral metallofullerenes: a decisive role of cooling gas and metal-carbon bonding.Nanoscale. 2016 Feb 14;8(6):3796-808. doi: 10.1039/c5nr08645k. Epub 2016 Jan 27. Nanoscale. 2016. PMID: 26815243 Free PMC article.
-
Modification of boron nitride nanocages by titanium doping results unexpectedly in exohedral complexes.Nat Commun. 2019 Oct 28;10(1):4908. doi: 10.1038/s41467-019-12877-0. Nat Commun. 2019. PMID: 31659166 Free PMC article.
References
-
- Kroto H. W., Heath J., O'Brien S., Curl R., Smalley R. Nature. 1985;318:162–163.
-
- Heath J. R., O'Brien S. C., Zhang Q., Liu Y., Curl R. F., Tittel F. K., Smalley R. E. J. Am. Chem. Soc. 1985;107:7779–7780.
-
- Dorn H. C., Stevenson S., Rice G., Glass T., Harich K., Cromer F., Jordan M. R., Craft J., Hadju E., Bible R., Olmstead M. M., Maitra K., Fisher A. J., Balch A. L. Nature. 1999;401:55–57.
-
- Rivera-Nazario D. M., Pinzón J. R., Stevenson S., Echegoyen L. A. J. Phys. Org. Chem. 2013;26:194–205.
-
- Chaur M., Valencia R., Rodríguez-Fortea A., Poblet J. M., Echegoyen L. Angew. Chem., Int. Ed. 2009;48:1425–1428. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

