Robust antibody and CD8+ T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection
- PMID: 28936360
- PMCID: PMC5603302
- DOI: 10.1038/s41541-017-0011-y
Robust antibody and CD8+ T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection
Abstract
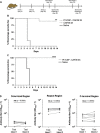
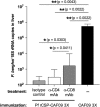
Despite several decades of extensive research, the development of a highly efficacious malaria vaccine has yet to be accomplished. While the RTS,S malaria vaccine candidate shows the potential to prevent a substantial number of clinical malaria cases, significant improvements in protective efficacy are still needed. Multiple studies have shown that RTS,S induces protective antibody and CD4+ T-cell responses, but limited or negligible CD8+ T cells. In this study, we evaluated the immunogenicity and protective capacity of full-length recombinant P. falciparum circumsporozoite protein (CSP) administered with the novel cationic liposomal adjuvant system CAF09. Using newly developed transgenic rodent malaria parasites expressing the full-length P. falciparum CSP, we demonstrate that this liposome-based protein-in-adjuvant formulation is capable of inducing robust antibody and CD8+ T-cell responses that strongly inhibit parasite infection and development of liver stages, conferring durable sterilizing immunity. These findings underscore the potential of liposome-based adjuvants for inducing robust humoral and CD8+ T-cell responses and warrant further studies toward the development of novel subunit vaccine formulations with this adjuvant system.
Keywords: CD8+ T cells; P. falciparum; adjuvant; antibodies; circumsporozoite protein; malaria; vaccine.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

