Acid Stress Response Mechanisms of Group B Streptococci
- PMID: 28936424
- PMCID: PMC5594096
- DOI: 10.3389/fcimb.2017.00395
Acid Stress Response Mechanisms of Group B Streptococci
Abstract
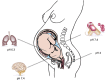
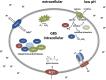
Group B streptococcus (GBS) is a leading cause of neonatal mortality and morbidity in the United States and Europe. It is part of the vaginal microbiota in up to 30% of pregnant women and can be passed on to the newborn through perinatal transmission. GBS has the ability to survive in multiple different host niches. The pathophysiology of this bacterium reveals an outstanding ability to withstand varying pH fluctuations of the surrounding environments inside the human host. GBS host pathogen interations include colonization of the acidic vaginal mucosa, invasion of the neutral human blood or amniotic fluid, breaching of the blood brain barrier as well as survival within the acidic phagolysosomal compartment of macrophages. However, investigations on GBS responses to acid stress are limited. Technologies, such as whole genome sequencing, genome-wide transcription and proteome mapping facilitate large scale identification of genes and proteins. Mechanisms enabling GBS to cope with acid stress have mainly been studied through these techniques and are summarized in the current review.
Keywords: Streptococcus agalactiae; acid resistance; low pH; molecular mechanisms; stress response.
Figures
Similar articles
-
Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs.Antonie Van Leeuwenhoek. 2012 Mar;101(3):677-82. doi: 10.1007/s10482-011-9666-y. Epub 2011 Oct 29. Antonie Van Leeuwenhoek. 2012. PMID: 22038130
-
Whole-Genome Comparison Uncovers Genomic Mutations between Group B Streptococci Sampled from Infected Newborns and Their Mothers.J Bacteriol. 2015 Oct;197(20):3354-66. doi: 10.1128/JB.00429-15. Epub 2015 Aug 17. J Bacteriol. 2015. PMID: 26283765 Free PMC article.
-
Group B streptococcus alters properties of vaginal epithelial cells in pregnant women.Am J Obstet Gynecol. 2016 Mar;214(3):383.e1-5. doi: 10.1016/j.ajog.2015.12.053. Am J Obstet Gynecol. 2016. PMID: 26928153
-
The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen.J Mol Biol. 2019 Jul 26;431(16):2914-2931. doi: 10.1016/j.jmb.2019.01.035. Epub 2019 Jan 31. J Mol Biol. 2019. PMID: 30711542 Free PMC article. Review.
-
Preventing neonatal group B streptococcal infection. Intrapartum antibiotic prophylaxis in some high-risk situations.Prescrire Int. 2011 Mar;20(114):72-7. Prescrire Int. 2011. PMID: 21648230 Review.
Cited by
-
Two-Component Signal Transduction Systems in the Human Pathogen Streptococcus agalactiae.Infect Immun. 2020 Jun 22;88(7):e00931-19. doi: 10.1128/IAI.00931-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 31988177 Free PMC article. Review.
-
Alterations of the vaginal microbiome in healthy pregnant women positive for group B Streptococcus colonization during the third trimester.BMC Microbiol. 2022 Dec 21;22(1):313. doi: 10.1186/s12866-022-02730-8. BMC Microbiol. 2022. PMID: 36544085 Free PMC article.
-
An Acid Up-Regulated Surface Protein of Lactobacillus paracasei Strain GCRL 46 is Phylogenetically Related to the Secreted Glucan- (GpbB) and Immunoglobulin-Binding (SibA) Protein of Pathogenic Streptococci.Int J Mol Sci. 2019 Mar 31;20(7):1610. doi: 10.3390/ijms20071610. Int J Mol Sci. 2019. PMID: 30935131 Free PMC article.
-
The LuxS/AI-2 Quorum-Sensing System of Streptococcus pneumoniae Is Required to Cause Disease, and to Regulate Virulence- and Metabolism-Related Genes in a Rat Model of Middle Ear Infection.Front Cell Infect Microbiol. 2018 May 4;8:138. doi: 10.3389/fcimb.2018.00138. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29780750 Free PMC article.
-
RECTA: Regulon Identification Based on Comparative Genomics and Transcriptomics Analysis.Genes (Basel). 2018 May 30;9(6):278. doi: 10.3390/genes9060278. Genes (Basel). 2018. PMID: 29849014 Free PMC article.
References
-
- Acikgoz Z. C., Gamberzade S., Gocer S., Ceylan P. (2005). Inhibitor effect of vaginal lactobacilli on group B streptococci. Mikrobiyol. Bul. 39, 17–23. - PubMed
-
- Anderson E. S., Paulley J. T., Gaines J. M., Valderas M. W., Martin D. W., Menscher E., et al. . (2009). The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect. Immun. 77, 3466–3474. 10.1128/IAI.00444-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

