Comparison of Diabetic and Non-diabetic Human Leukocytic Responses to Different Capsule Types of Klebsiella pneumoniae Responsible for Causing Pyogenic Liver Abscess
- PMID: 28936426
- PMCID: PMC5594087
- DOI: 10.3389/fcimb.2017.00401
Comparison of Diabetic and Non-diabetic Human Leukocytic Responses to Different Capsule Types of Klebsiella pneumoniae Responsible for Causing Pyogenic Liver Abscess
Abstract
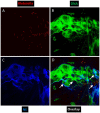
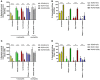
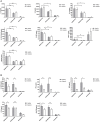
The major risk factor for Klebsiella liver abscess (KLA) is type 2 diabetes mellitus (DM), but the immunological mechanisms involved in the increased susceptibility are poorly defined. We investigated the responses of neutrophils and peripheral blood mononuclear cells (PBMCs) to hypervirulent Klebsiella pneumoniae (hvKP), the causative agent of KLA. DNA and myeloperoxidase levels were elevated in the plasma of KLA patients compared to uninfected individuals indicating neutrophil activation, but diabetic status had no effect on these neutrophil extracellular trap (NET) biomarkers in both subject groups. Clinical hvKP isolates universally stimulated KLA patient neutrophils to produce NETs ex vivo, regardless of host diabetic status. Ability of representative capsule types (K1, K2, and non-K1/K2 strains) to survive intra- and extra-cellular killing by type 2 DM and healthy neutrophils was subsequently examined. Key findings were: (1) type 2 DM and healthy neutrophils exhibited comparable total, phagocytic, and NETs killing against hvKP, (2) phagocytic and NETs killing were equally effective against hvKP, and (3) hypermucoviscous K1 and K2 strains were more resistant to total, phagocytic, and NETs killing compared to the non-mucoviscous, non-K1/K2 strain. The cytokine response and intracellular killing ability of type 2 DM as well as healthy PBMCs upon encounter with the different capsule types was also examined. Notably, the IL-12-IFNγ axis and its downstream chemokines MIG, IP-10, and RANTES were produced at slightly lower levels by type 2 DM PBMCs than healthy PBMCs in response to representative K1 and non-K1/K2 strains. Furthermore, type 2 DM PBMCs have a mild defect in its ability to control hvKP replication relative to healthy PBMCs. In summary, our work demonstrates that type 2 DM does not overtly impact neutrophil intra- and extra-cellular killing of hvKP, but may influence cytokine/chemokine production and intracellular killing by PBMCs.
Keywords: Klebsiella pneumoniae; cytokines; hypervirulent; liver abscess; neutrophil extracellular trap; neutrophils; peripheral blood mononuclear cells; type 2 diabetes.
Figures



Similar articles
-
Increasing opsonizing and killing effect of serum from patients with recurrent K1 Klebsiella pneumoniae liver abscess.J Microbiol Immunol Infect. 2012 Apr;45(2):141-6. doi: 10.1016/j.jmii.2011.12.006. Epub 2012 Mar 21. J Microbiol Immunol Infect. 2012. PMID: 22444545
-
High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess.Microbes Infect. 2004 Nov;6(13):1191-8. doi: 10.1016/j.micinf.2004.06.003. Microbes Infect. 2004. PMID: 15488738
-
Resistance of hypervirulent Klebsiella pneumoniae to both intracellular and extracellular killing of neutrophils.PLoS One. 2017 Mar 10;12(3):e0173638. doi: 10.1371/journal.pone.0173638. eCollection 2017. PLoS One. 2017. PMID: 28282434 Free PMC article.
-
Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives.J Intern Med. 2020 Mar;287(3):283-300. doi: 10.1111/joim.13007. Epub 2019 Nov 21. J Intern Med. 2020. PMID: 31677303 Free PMC article. Review.
-
Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics.Am J Gastroenterol. 2005 Feb;100(2):322-31. doi: 10.1111/j.1572-0241.2005.40310.x. Am J Gastroenterol. 2005. PMID: 15667489 Review.
Cited by
-
The Pathogenic Bacteria of Deep Neck Infection in Patients with Type 1 Diabetes, Type 2 Diabetes, and Without Diabetes from Chang Gung Research Database.Microorganisms. 2021 Sep 29;9(10):2059. doi: 10.3390/microorganisms9102059. Microorganisms. 2021. PMID: 34683380 Free PMC article.
-
Hypervirulent Klebsiella pneumoniae.Infect Drug Resist. 2023 Aug 11;16:5243-5249. doi: 10.2147/IDR.S418523. eCollection 2023. Infect Drug Resist. 2023. PMID: 37589017 Free PMC article. Review.
-
A Molecular Interaction Map of Klebsiella pneumoniae and Its Human Host Reveals Potential Mechanisms of Host Cell Subversion.Front Microbiol. 2021 Feb 18;12:613067. doi: 10.3389/fmicb.2021.613067. eCollection 2021. Front Microbiol. 2021. PMID: 33679637 Free PMC article.
-
Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: implications in combating infections.Hum Cell. 2023 Jul;36(4):1265-1282. doi: 10.1007/s13577-023-00905-7. Epub 2023 Apr 28. Hum Cell. 2023. PMID: 37115481 Free PMC article. Review.
-
The Bacterial Compositions of Nasal Septal Abscess in Patients with or without Diabetes.Life (Basel). 2022 Dec 13;12(12):2093. doi: 10.3390/life12122093. Life (Basel). 2022. PMID: 36556459 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

