Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective
- PMID: 28936429
- PMCID: PMC5581366
- DOI: 10.1007/s40336-016-0213-8
Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective
Abstract
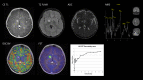
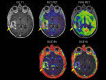
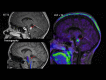
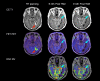
Purpose: Magnetic resonance imaging (MRI) plays a key role in neurooncology, i.e., for diagnosis, treatment evaluation and detection of recurrence. However, standard MRI cannot always separate malignant tissue from other pathologies or treatment-induced changes. Advanced MRI techniques such as diffusion-weighted imaging, perfusion imaging and spectroscopy show promising results in discriminating malignant from benign lesions. Further, supplemental imaging with amino acid positron emission tomography (PET) has been shown to increase accuracy significantly and is used routinely at an increasing number of sites. Several centers are now implementing hybrid PET/MRI systems allowing for multiparametric imaging, combining conventional MRI with advanced MRI and amino acid PET imaging. Neurooncology is an obvious focus area for PET/MR imaging.
Methods: Based on the literature and our experience from more than 300 PET/MRI examinations of brain tumors with 18F-fluoro-ethyl-tyrosine, the clinical use of PET/MRI in adult and pediatric neurooncology is critically reviewed.
Results: Although the results are increasingly promising, the added value and range of indications for multiparametric imaging with PET/MRI are yet to be established. Robust solutions to overcome the number of issues when using a PET/MRI scanner are being developed, which is promising for a more routine use in the future.
Conclusions: In a clinical setting, a PET/MRI scan may increase accuracy in discriminating recurrence from treatment changes, although sequential same-day imaging on separate systems will often constitute a reliable and cost-effective alternative. Pediatric patients who require general anesthesia will benefit the most from simultaneous PET and MR imaging.
Keywords: 18F-fluoro-ethyl-tyrosine; Brain tumor; FET; Glioma; Multiparametric imaging; PET/MRI; Pediatric.
Conflict of interest statement
All authors (ML, OMH, MLJ, VAL and IL) declare no conflicts of interest.
For imaging obtained in brain imaging trials, all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in each study.
Figures
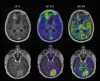

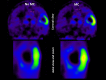
Similar articles
-
Early Postoperative 18F-FET PET/MRI for Pediatric Brain and Spinal Cord Tumors.J Nucl Med. 2019 Aug;60(8):1053-1058. doi: 10.2967/jnumed.118.220293. Epub 2019 Jan 25. J Nucl Med. 2019. PMID: 30683767
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Simultaneous whole body (18)F-fluorodeoxyglucose positron emission tomography magnetic resonance imaging for evaluation of pediatric cancer: Preliminary experience and comparison with (18)F-fluorodeoxyglucose positron emission tomography computed tomography.World J Radiol. 2016 Mar 28;8(3):322-30. doi: 10.4329/wjr.v8.i3.322. World J Radiol. 2016. PMID: 27028112 Free PMC article.
-
The Utility of Conventional Amino Acid PET Radiotracers in the Evaluation of Glioma Recurrence also in Comparison with MRI.Diagnostics (Basel). 2022 Mar 29;12(4):844. doi: 10.3390/diagnostics12040844. Diagnostics (Basel). 2022. PMID: 35453892 Free PMC article. Review.
-
Combined Amino Acid Positron Emission Tomography and Advanced Magnetic Resonance Imaging in Glioma Patients.Cancers (Basel). 2019 Jan 29;11(2):153. doi: 10.3390/cancers11020153. Cancers (Basel). 2019. PMID: 30699942 Free PMC article. Review.
Cited by
-
Diagnostic accuracy and clinical impact of [18F]FET PET in childhood CNS tumors.Neuro Oncol. 2021 Dec 1;23(12):2107-2116. doi: 10.1093/neuonc/noab096. Neuro Oncol. 2021. PMID: 33864083 Free PMC article.
-
The Molecular Effects of Ionizing Radiations on Brain Cells: Radiation Necrosis vs. Tumor Recurrence.Diagnostics (Basel). 2019 Sep 24;9(4):127. doi: 10.3390/diagnostics9040127. Diagnostics (Basel). 2019. PMID: 31554255 Free PMC article. Review.
-
FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century.Front Oncol. 2022 May 25;12:854658. doi: 10.3389/fonc.2022.854658. eCollection 2022. Front Oncol. 2022. PMID: 35692767 Free PMC article. Review.
-
Maximizing the use of batch production of 18F-FDOPA for imaging of brain tumors to increase availability of hybrid PET/MR imaging in clinical setting.Neurooncol Pract. 2020 Oct 14;8(1):91-97. doi: 10.1093/nop/npaa065. eCollection 2021 Feb. Neurooncol Pract. 2020. PMID: 33664973 Free PMC article.
-
Diagnostic Performance of PET and Perfusion-Weighted Imaging in Differentiating Tumor Recurrence or Progression from Radiation Necrosis in Posttreatment Gliomas: A Review of Literature.AJNR Am J Neuroradiol. 2020 Sep;41(9):1550-1557. doi: 10.3174/ajnr.A6685. Epub 2020 Aug 27. AJNR Am J Neuroradiol. 2020. PMID: 32855194 Free PMC article. Review.
References
-
- Rachinger W, Goetz C, Popperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505–511. doi: 10.1227/01.NEU.0000171642.49553.B0. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
