Porcine Esophageal Submucosal Gland Culture Model Shows Capacity for Proliferation and Differentiation
- PMID: 28936470
- PMCID: PMC5602779
- DOI: 10.1016/j.jcmgh.2017.07.005
Porcine Esophageal Submucosal Gland Culture Model Shows Capacity for Proliferation and Differentiation
Abstract
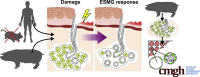
Background & aims: Although cells comprising esophageal submucosal glands (ESMGs) represent a potential progenitor cell niche, new models are needed to understand their capacity to proliferate and differentiate. By histologic appearance, ESMGs have been associated with both overlying normal squamous epithelium and columnar epithelium. Our aim was to assess ESMG proliferation and differentiation in a 3-dimensional culture model.
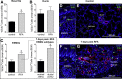
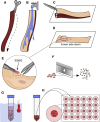
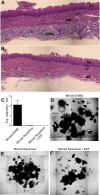
Methods: We evaluated proliferation in human ESMGs from normal and diseased tissue by proliferating cell nuclear antigen immunohistochemistry. Next, we compared 5-ethynyl-2'-deoxyuridine labeling in porcine ESMGs in vivo before and after esophageal injury with a novel in vitro porcine organoid ESMG model. Microarray analysis of ESMGs in culture was compared with squamous epithelium and fresh ESMGs.
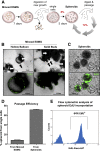
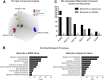
Results: Marked proliferation was observed in human ESMGs of diseased tissue. This activated ESMG state was recapitulated after esophageal injury in an in vivo porcine model, ESMGs assumed a ductal appearance with increased proliferation compared with control. Isolated and cultured porcine ESMGs produced buds with actively cycling cells and passaged to form epidermal growth factor-dependent spheroids. These spheroids were highly proliferative and were passaged multiple times. Two phenotypes of spheroids were identified: solid squamous (P63+) and hollow/ductal (cytokeratin 7+). Microarray analysis showed spheroids to be distinct from parent ESMGs and enriched for columnar transcripts.
Conclusions: Our results suggest that the activated ESMG state, seen in both human disease and our porcine model, may provide a source of cells to repopulate damaged epithelium in a normal manner (squamous) or abnormally (columnar epithelium). This culture model will allow the evaluation of factors that drive ESMGs in the regeneration of injured epithelium. The raw microarray data have been uploaded to the National Center for Biotechnology Information Gene Expression Omnibus (accession number: GSE100543).
Keywords: 3D Culture; 3D, 3-dimensional; ANOVA, analysis of variance; Acinar Ductal Metaplasia; Adult Stem Cell; BE, Barrett’s esophagus; Barrett’s Esophagus; CK7, cytokeratin 7; DMSO, dimethyl sulfoxide; EAC, esophageal adenocarcinoma; EGF, epidermal growth factor; ESMG, esophageal submucosal gland; EdU, 5-ethynyl-2′-deoxyuridine; Esophagus; IHC, immunohistochemistry; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; RFA, radiofrequency ablation.
Figures




References
-
- Shi L., Der R., Ma Y., Peters J., Demeester T., Chandrasoma P. Gland ducts and multilayered epithelium in mucosal biopsies from gastroesophageal-junction region are useful in characterizing esophageal location. Dis Esophagus. 2005;18:87–92. - PubMed
-
- van Nieuwenhove Y., Destordeur H., Willems G. Spatial distribution and cell kinetics of the glands in the human esophageal mucosa. Eur J Morphol. 2001;39:163–168. - PubMed
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Neymann N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, eds. SEER cancer statistics review, 1975-2011 Bethesda, MD: National Cancer Institute; 2014 based on November 2013 SEER data submission, posted to the SEER web site, April 4. Available from: http://seer.cancer.gov/csr/1975_2011. Accessed October 17, 2014.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

