Platelet dense granules begin to selectively accumulate mepacrine during proplatelet formation
- PMID: 28936487
- PMCID: PMC5604861
- DOI: 10.1182/bloodadvances.2017006726
Platelet dense granules begin to selectively accumulate mepacrine during proplatelet formation
Abstract
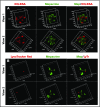
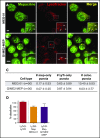
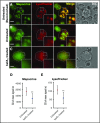
Platelet dense granules (DGs) are storage organelles for calcium ions, small organic molecules such as ADP and serotonin, and larger polyphosphates that are secreted upon platelet stimulation to enhance platelet activation, adhesion, and stabilization at sites of vascular damage. DGs are thought to fully mature within megakaryocytes (MKs) prior to platelet formation. Here we challenge this notion by exploiting vital fluorescent dyes to distinguish mildly acidic DGs from highly acidic compartments by microscopy in platelets and MKs. In isolated primary mouse platelets, compartments labeled by mepacrine - a fluorescent weak base that accumulates in DGs - are readily distinguishable from highly acidic compartments, likely lysosomes, that are labeled by the acidic pH indicator, LysoTracker, and from endolysosomes and alpha granules labeled by internalized and partially digested DQ™ BSA. By contrast, in murine fetal liver- and human CD34+ cell-derived MKs and the megakaryocytoid cell lines, MEG-01 and differentiated G1ME2, labeling by mepacrine overlapped nearly completely with labeling by LysoTracker and partially with labeling by DQ™ BSA. Mepacrine labeling in G1ME2-derived MKs was fully sensitive to proton ATPase inhibitors, but was only partially sensitive in platelets. These data indicate that mepacrine in MKs accumulates as a weak base in endolysosomes but is likely pumped into or retained in separate DGs in platelets. Fluorescent puncta that labeled uniquely for mepacrine were first evident in G1ME2-derived proplatelets, suggesting that DGs undergo a maturation step that initiates in the final stages of MK differentiation.
Keywords: dense granule; live cell imaging; lysosome; lysosome-related organelle; megakaryocyte; platelets; proplatelet.
Conflict of interest statement
DISCLOSURE OF CONFLICTS OF INTEREST The authors declare no competing financial interests.
Figures




References
-
- Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015;13(12):2141-2151. - PubMed
-
- Wei AH, Li W. Hermansky-Pudlak syndrome: pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013;26(2):176-192. - PubMed
-
- Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(Suppl 1):76-91. - PubMed
-
- Seward SL Jr, Gahl WA. Hermansky-Pudlak syndrome: health care throughout life. Pediatrics. 2013;132(1):153-160. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

