Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome
- PMID: 28936695
- PMCID: PMC5633625
- DOI: 10.1007/s00134-017-4912-z
Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome
Abstract
Purpose: Experimental animal models of acute respiratory distress syndrome (ARDS) have shown that the updated airway pressure release ventilation (APRV) methodologies may significantly improve oxygenation, maximize lung recruitment, and attenuate lung injury, without circulatory depression. This led us to hypothesize that early application of APRV in patients with ARDS would allow pulmonary function to recover faster and would reduce the duration of mechanical ventilation as compared with low tidal volume lung protective ventilation (LTV).
Methods: A total of 138 patients with ARDS who received mechanical ventilation for <48 h between May 2015 to October 2016 while in the critical care medicine unit (ICU) of the West China Hospital of Sichuan University were enrolled in the study. Patients were randomly assigned to receive APRV (n = 71) or LTV (n = 67). The settings for APRV were: high airway pressure (Phigh) set at the last plateau airway pressure (Pplat), not to exceed 30 cmH2O) and low airway pressure ( Plow) set at 5 cmH2O; the release phase (Tlow) setting adjusted to terminate the peak expiratory flow rate to ≥ 50%; release frequency of 10-14 cycles/min. The settings for LTV were: target tidal volume of 6 mL/kg of predicted body weight; Pplat not exceeding 30 cmH2O; positive end-expiratory pressure (PEEP) guided by the PEEP-FiO2 table according to the ARDSnet protocol. The primary outcome was the number of days without mechanical ventilation from enrollment to day 28. The secondary endpoints included oxygenation, Pplat, respiratory system compliance, and patient outcomes.
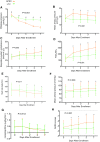
Results: Compared with the LTV group, patients in the APRV group had a higher median number of ventilator-free days {19 [interquartile range (IQR) 8-22] vs. 2 (IQR 0-15); P < 0.001}. This finding was independent of the coexisting differences in chronic disease. The APRV group had a shorter stay in the ICU (P = 0.003). The ICU mortality rate was 19.7% in the APRV group versus 34.3% in the LTV group (P = 0.053) and was associated with better oxygenation and respiratory system compliance, lower Pplat, and less sedation requirement during the first week following enrollment (P < 0.05, repeated-measures analysis of variance).
Conclusions: Compared with LTV, early application of APRV in patients with ARDS improved oxygenation and respiratory system compliance, decreased Pplat and reduced the duration of both mechanical ventilation and ICU stay.
Keywords: Acute respiratory distress syndrome; Airway pressure release ventilation; Low tidal volume; Spontaneous breathing.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethics committee of West China Hospital of Sichuan University in accordance with the Helsinki Declaration. Written informed consent was obtained from the patients’ authorized surrogates. The clinical trial registration number was NCT02639364.
Conflicts of interest
All authors declare that they do not have any conflict of interest relevant to this study.
Figures



Comment in
-
Early application of airway pressure release ventilation in acute respiratory distress syndrome: a therapy for all?Intensive Care Med. 2018 Jan;44(1):135-136. doi: 10.1007/s00134-017-4983-x. Epub 2017 Nov 9. Intensive Care Med. 2018. PMID: 29124317 No abstract available.
-
Airway pressure release ventilation: a step forward?Intensive Care Med. 2018 Feb;44(2):272. doi: 10.1007/s00134-017-5012-9. Epub 2017 Dec 11. Intensive Care Med. 2018. PMID: 29230522 No abstract available.
-
Airway pressure release ventilation (APRV): do good things come to those who can wait?J Thorac Dis. 2018 Feb;10(2):667-669. doi: 10.21037/jtd.2018.01.107. J Thorac Dis. 2018. PMID: 29607131 Free PMC article. No abstract available.
-
Airway pressure release ventilation in patients with acute respiratory distress syndrome: not yet, we still need more data!J Thorac Dis. 2018 Feb;10(2):670-673. doi: 10.21037/jtd.2017.11.143. J Thorac Dis. 2018. PMID: 29607132 Free PMC article. No abstract available.
-
APRV for ARDS: the complexities of a mode and how it affects even the best trials.J Thorac Dis. 2018 Apr;10(Suppl 9):S1058-S1063. doi: 10.21037/jtd.2018.03.156. J Thorac Dis. 2018. PMID: 29850185 Free PMC article. No abstract available.
-
Estimation of true driving pressure during airway pressure release ventilation : Discussion on "Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome".Intensive Care Med. 2018 Aug;44(8):1364-1365. doi: 10.1007/s00134-018-5250-5. Epub 2018 Jun 15. Intensive Care Med. 2018. PMID: 29947887 No abstract available.
-
Airway pressure release ventilation in acute respiratory distress syndrome: children are not miniature adults.Intensive Care Med. 2018 Sep;44(9):1595-1596. doi: 10.1007/s00134-018-5337-z. Epub 2018 Aug 6. Intensive Care Med. 2018. PMID: 30083990 No abstract available.
References
-
- Cressoni M, Chiumello D, Algieri I, Brioni M, Chiurazzi C, Colombo A, Colombo A, Crimella F, Guanziroli M, Tomic I, Tonetti T, Luca Vergani G, Carlesso E, Gasparovic V, Gattinoni L. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43(5):603–611. doi: 10.1007/s00134-017-4754-8. - DOI - PubMed
-
- Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160–166. doi: 10.1164/rccm.200607-915OC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

