Misfolding and aggregation of nascent proteins: a novel mode of toxic cadmium action in vivo
- PMID: 28936749
- PMCID: PMC5778182
- DOI: 10.1007/s00294-017-0748-x
Misfolding and aggregation of nascent proteins: a novel mode of toxic cadmium action in vivo
Abstract
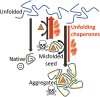
Cadmium is a highly poisonous metal and a human carcinogen, but the molecular mechanisms underlying its cellular toxicity are not fully understood. Recent findings in yeast cells indicate that cadmium exerts its deleterious effects by inducing widespread misfolding and aggregation of nascent proteins. Here, we discuss this novel mode of toxic heavy metal action and propose a mechanism by which molecular chaperones may reduce the damaging effects of heavy metal ions on protein structures.
Keywords: Cadmium; Metal toxicity; Molecular chaperones; Protein aggregation; Protein folding; Saccharomyces cerevisiae.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

