Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health
- PMID: 28936943
- PMCID: PMC11687544
- DOI: 10.1128/microbiolspec.BAD-0019-2017
Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health
Abstract
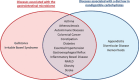
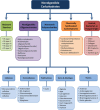
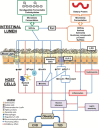
There is a clear association between the gastrointestinal (GI) microbiome and the development of chronic noncommunicable diseases, providing a rationale for the development of strategies that target the GI microbiota to improve human health. In this article, we discuss the potential of supplementing the human diet with nondigestible fermentable carbohydrates (NDFCs) to modulate the composition, structure, diversity, and metabolic potential of the GI microbiome in an attempt to prevent or treat human disease. The current concepts by which NDFCs can be administered to humans, including prebiotics, fermentable dietary fibers, and microbiota-accessible carbohydrates, as well as the mechanisms by which these carbohydrates exert their health benefits, are discussed. Epidemiological research presents compelling evidence for the health effects of NDFCs, with clinical studies providing further support for some of these benefits. However, rigorously designed human intervention studies with well-established clinical markers and microbial endpoints are still essential to establish (i) the clinical efficiency of specific NDFCs, (ii) the causal role of the GI microbiota in these effects, (iii) the underlying mechanisms involved, and (iv) the degree by which inter-individual differences between GI microbiomes influence these effects. Such studies would provide the mechanistic understanding needed for a systematic application of NDFCs to improve human health via GI microbiota modulation while also allowing the personalization of these dietary strategies.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

